
ADP


AT
4 Carbon compound

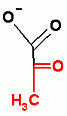
pyruvate carboxylase
Yeasts
Ethanol fermentation: Beers, Wines The CO2 released is used to raise bread


Lactate fermentation:(overworked muscle cells, red blood cells, yogurt making (Lactobacillus acidophilus))
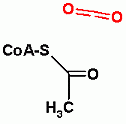
Glycolysis only, ATP Dependent Group Transfer Reaction
Gluconeogenesis Only, Hydrolysis Reaction
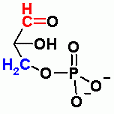
Readily Reversible, Both Glycolysis and Gluconeogenesis, Isomerization Reaction
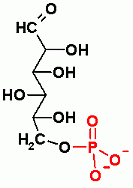
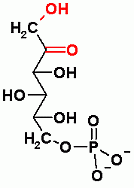
Reversible, Both Glycolysis and Gluconeogenesis, C-C bond aldol Reaction. Standard State Thermodynamics (ΔGo') favors gluconeogenesis, but under normal physiological conditions the ΔG favors glycolysis
Both glycolysis and gluconeogenesis. Two sequential reaction types on the same enzyme active site.
Both glycolysis and gluconeogenesis. ATP Dependent group Transfer Reaction. Unlike the two similar ATP dependent reactions above, this reaction is readily reversible.
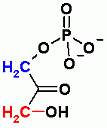
Both glycolysis and gluconeogenesis. Group Transfer reaction. While the substrate and product are isomers this is NOT an isomerization reaction
Both glycolysis and gluconeogenesis. Dehydration Reaction- in the direction of glycolysis the equivalent of water is removed between C2 and C3. In the direction of gluconeogenesis water is added across the C2-C3 double bond.
Gluconeogenesis only. Two successive steps
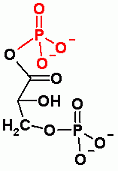
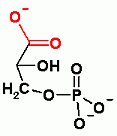
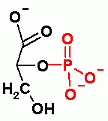
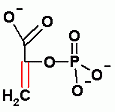
Gluconeogenesis only. Aldol reaction to add CO2. In order to add CO2 ATP must be expended. Check out the page to see how and why.

| Enzymes exclusive to Glycolysis | Enzymes Shared between the two pathways | Enzymes exclusive to gluconeogenesis |
| Get a "quick summary" by hovering over a link. Follow the links on the enzyme name to learn more about its chemistry and its catalytic mechanism. There are also some rationales about how the two pathways fit together, which reactions are the same and which must be different to maintain favorable standard free energies for both overall pathways. | ||||
 | ||||
ADP | | |||
 | ||||
 | ||||
 | ||||
  | ||||
 | ||||
 | ||||
 | ||||
 |
||||
 |
||||
| Compare the glycolysis, uncatalyzed and gluconeogenesis reactions side by side | AT | 4 Carbon compound | ||
 |
pyruvate carboxylase | |||
 | ||||
| glycolysis "officially" ends here, but 1 of the 3 steps below are required! | ||||
Yeasts Ethanol fermentation: Beers, Wines The CO2 released is used to raise bread |  |  | Lactate fermentation:(overworked muscle cells, red blood cells, yogurt making (Lactobacillus acidophilus)) | |
 |  | |||
 | ||||
| Acetyl-CoA is NOT an end point of fermentative processes like ethanol or lactate. This takes place in mitochondria and can lead to oxidative glucose metabolism. (the subject of module 7). It is a compound that can be used in many processes including synthesis of some amino acids and synthesis of all fatty acids. | ||||
Notes:

In places where the arrows are not vertical... this simply denotes where one pathway uses two reactions while the other pathway utilizes only a single reaction.