|
Enzyme Name |
Hexokinase |
|
|
Reaction Catalyzed |
ATP dependent phosphorylation of Glucose
|
|
|
Reaction Type |
Group Transfer |
|
|
Pathway involvement |
Glycolysis ONLY |
Hexokinase is not part of the gluconeogenesis pathway. In gluconeogenesis a separate enzyme (Glucose-6-Phosphatase) hydrolyzes a phosphate from Glucose-6P and is not ATP dependent. Rather it performs a simple hydrolysis reaction to cleave off phosphate. |
|
Cofactors/Cosubstrates |
ATP is a cosubstrate; ADP is a coproduct | cosubstrate/coproduct = molecules that enter and leave the active site along with the substrate/product and are generally altered in the reaction as well cofactors = nonprotein molecules that are required for a reaction that are generally a permanent part of the enzyme. These remain unaltered after the reaction is complete. |

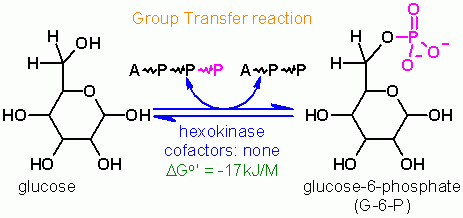

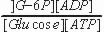

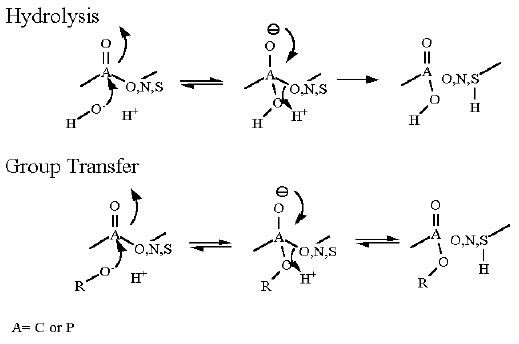
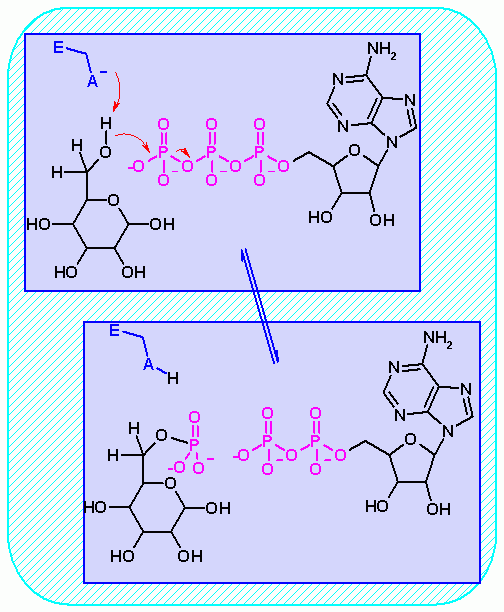
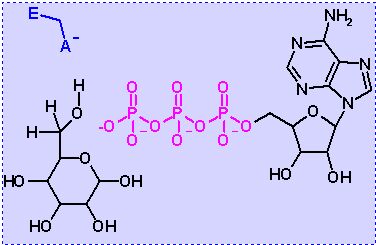 Hexokinase. Animation of an Hexokinase reaction Blue: represents the enzyme. THe EA- is the Aspartate from the enzyme active site in it's basic (deprotonated) state. "Start" begins an animation of the group transfer reaction. It proceeds through the reaction in the "forward" direction and then "backwards" again. Note how the enzyme is involved. "+" increases speed while "-" decreases the animation speed. You may also step through the reaction using "next" or "previous"
Hexokinase. Animation of an Hexokinase reaction Blue: represents the enzyme. THe EA- is the Aspartate from the enzyme active site in it's basic (deprotonated) state. "Start" begins an animation of the group transfer reaction. It proceeds through the reaction in the "forward" direction and then "backwards" again. Note how the enzyme is involved. "+" increases speed while "-" decreases the animation speed. You may also step through the reaction using "next" or "previous"