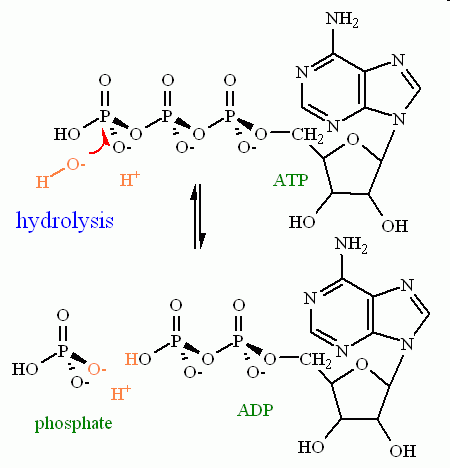
Hydrolysis. Cleavage of a bond by with addition of water. The bond to be cleaved must adhere to the following parameters:
The atom attacked by the HO- or nucleophile must be either C,P,S that has a double bond to either O, N or S. The leaving atom (the atom that moves away from the initial compound) must be O,N,S.
Hydrolysis/group transfer reactions CANNOT cleave a C-C bond!
See the arrow pushing in motion for a hydrolysis reaction
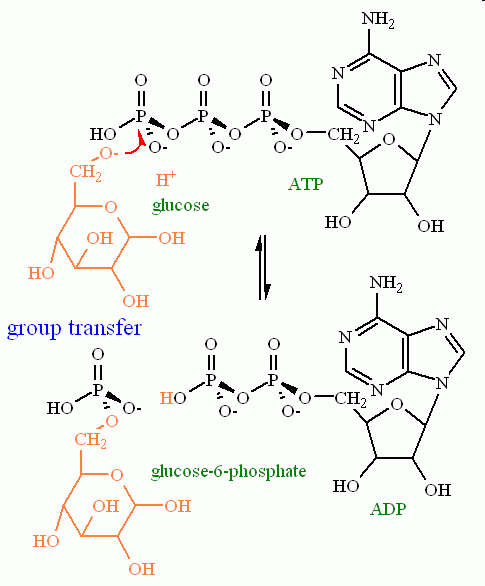
Group transfer. Group transfer follows the same rules as hydrolysis but the attacking nucleophile is not HO- but rather RO- Where R represents something other than H.
See the arrow pushing in motion for a group transfer reaction
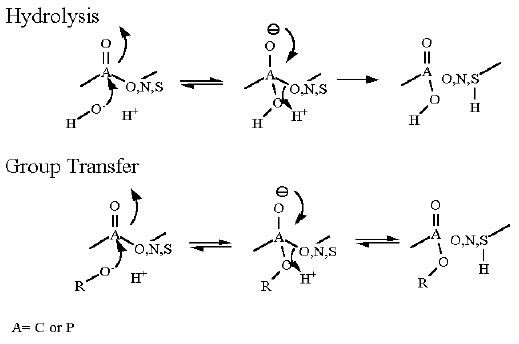
Look at the similarities between these two reactions. You should see that the overall flow of the electrons is identical in both cases. To give a specific example, hydrolysis of ATP yields ADP and inorganic phosphate.
one example of Group Transfer with ATP would be with glucose (common simple sugar) as the attacking agent. Here the products on the right would be ADP (just like the hydrolysis reaction) and glucose-6-phosphate.
There is one MAJOR difference between these two similar reactions: Their thermodynamics. Thermodynamically, the hydrolysis reaction is ALWAYS favored to the side of the reaction with two products (ADP + phosphate in our example). The thermodynamics of the group transfer reaction, however, depends heavily on the nature of the functional group that is acting as the nucleophile. (More about this when we discuss the metabolic pathway in module 6).
examples using ATP