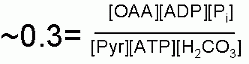|
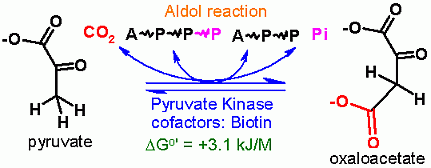
This is the first of two required enzyme to bypass pyruvate kinase. This one puts another carboxyl group on to pyruvate... so that the next one, phosphoenolpyruvate carboxykinase, can use the Free Energy of decarboxylation to drive the addition of phosphate to the "enol" group.
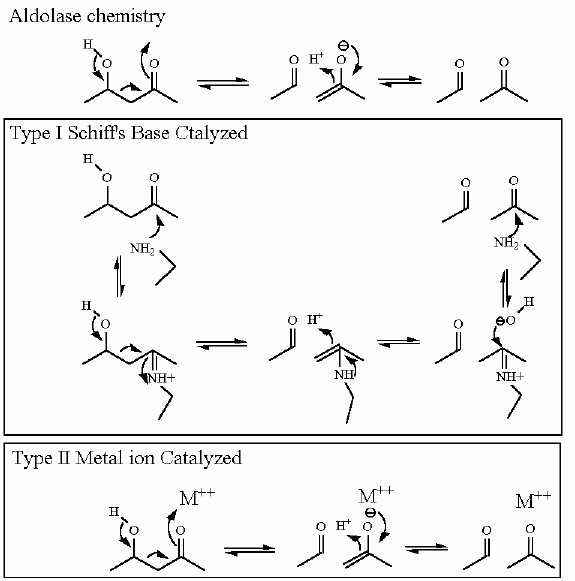
Carbon dioxide cannot be used as CO2 in mammalian systems for addition of a carbon by an Aldol reaction. First and foremost this is becasue we go to great lengths to keep our CO2 concentration low. There are two mechanisms:
- In the tissues, where CO2 is generated and therefore in relatively high concentration, an enzyme called Carbonic Anhydrase (CA) rapdidly converts CO2 to HCO3- + H+ by a reversible reaction with water.
- In the lungs, where CO2 concentration is low (atmosphereic concetrations), the same reaction catalyzed by the same enzyme runs the other way to generate CO2 so that we can exhale it.
The first two steps in this overall reaction are solely to prepare and capture HCO3- so that it is amenable to an aldol reaction with pyruvate. For chemical reasons (see comments below) bicabonate is a very poor substrate for an aldol reaction.
The first preparation step is to "activate" HC03- in a group transfer reaction with ATP. This generates the first intermediate, a carboxyl-phosphoanhydride. (notice the similarities of this to the phosphoanhydrides of ATP) and the first product, ADP.
The second capture step is to transfer the activated carbon dioxide to Biotin a cofactor that is covalently bound to the enzyme to generate carboxybiotin and phsophate. Carboxybiotin is kinetically stable and can now wait until pyruvate enters the active site.
The Aldol reaction between pyruvate and carboxybiotin generates the final product oxaloacetate as well as biotin (so that the enzyme can go through another cycle).
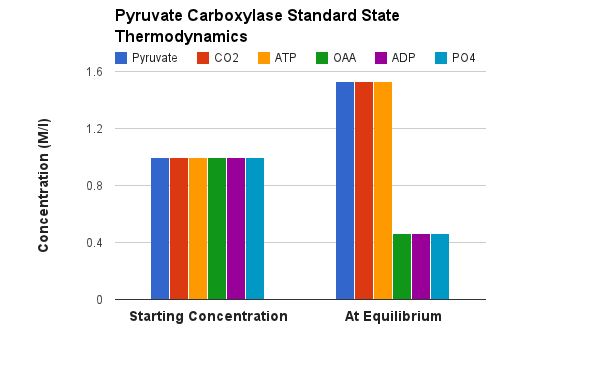
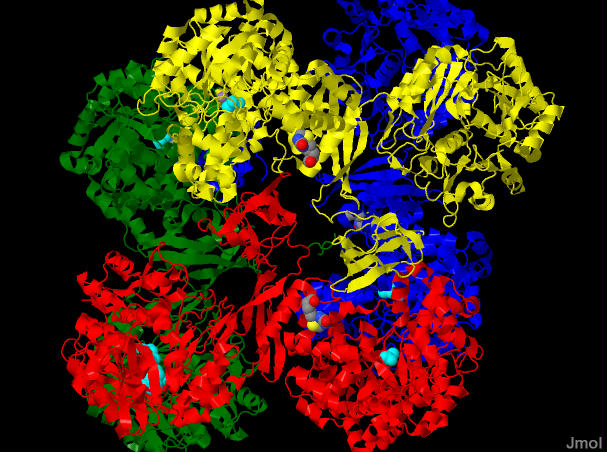
Notes on this mechanism. This is an extremely compelx enzyme.... even beyound the obvious that it combines ATP hydrolysis and an aldol reaction. The individual reactions occur in separate places in the enzyme. There is one active site for the generation of carboxybiotin and a separate active site where the carboxul group is tranferred to pyruvate. This necessitates a "transport" of the biotin from site to site. Indeed the biotin is covalently attached to a third spot (the bioton carrier domain) whose sole function is to move the biotin from site to site. It is even more complex than this... The active enzyme is a tetramer (four identical subunits) which we call a, b, c and d. The biotin carboxylation site and the pyruvate carboxylation site are on one polypeptide (b for instance) while the biotin is supplied from another subunit (a in this case). See pictures below.
Furthermore, the human enzyme needs to be "activated" in order to function. The essential Mn2+ ion is held in place in the enzyme by several amino acids. One of these is a modified lysine. As you might guess a positively charged lys is not a very good thing for holding onto a positively charged metal atom. This lysine must first be carbolylated in order to be able to hold onto the metal ion that initiates the aldol reaction. This is likely done by biotin.
|