

|
Reactions catalyzed |
|
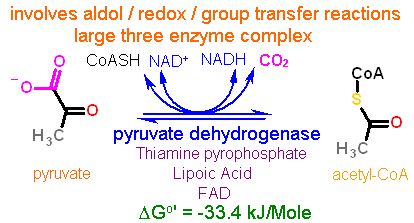
Glycolysis typically thought of ending with pyruvate, while the Krebs cycle is typically thought of as starting with acetyl-CoA. So there must be some conversion of pyruvate to acetyl-CoA. Indeed there is. The enzyme pyruvate dehydrogenase catalyzes this conversion. This conversion is quite complex and requires FIVE vitamin derived cofactors to complete.
A couple of these are old friends....
The full mechanism of pyruvate dehydrogenase is beyond the scope of this course but suffice it to say that this reaction requires the concerted efforts of three enzymes all held together in a giant complex to catalyze the conversion.
Three different types of chemistries are utilized overall:
The carbon that is cleaved off is removed as CO2. This is the first carbon lost as CO2 in the complete oxidation steps of glucose. Please recall that for every glucose that started glycolysis there are two acetyl-CoA. Thus two carbons have so far been completely oxidized.
Notice also the production of one NADH for each pyruvate oxidized as well.
I made a big deal in the first module about all reactions being reversible. While this one is theoretically reversible, for practical purposes it only runs toward acetyl-CoA. There are a couple very important reasons that combine to make this the case.
Acetyl-CoA is a central compound as many metabolic pathways branch off. Besides the Krebs cycle and a few others, fatty acids and several amino acids start here as well. Fatty acids (a main component of storage fats and cell membrane lipids) are made by putting acetyl-CoAs together end to end in an aldol reaction. (a couple of reductions for each acetyl group added and you have a fatty acid). Most Fatty acids contain an even number of carbons...now you know why.
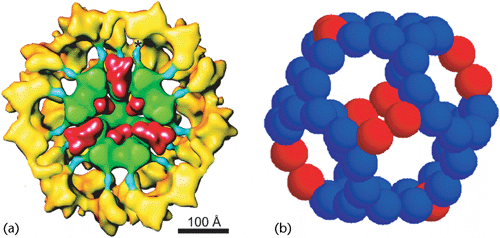 Two models of the mammalian PDH organization. (a) Cut-away model of a fully assembled PDH. Lipoic acid dehydrogenase molecules (red) are bound inside the pentagonal dodecahedron core formed by 60 inner domains of Acyl transferase (AT) (green). Pyruvate devarboxylase (yellow) is bound to the AT-core through the inner linkers of AT (blue) (Zhou et al., 2001). (b) A model of the inner core formed by AT. Twelve inner domains of BP (red) replace 12 inner domains of AT and bind with 48 inner domains of AT (blue) to form a 60-mer pentagonal dodecahedron (Hiromasa et al., 2004).Lioubov G Korotchkina, SUNY at Buffalo, Buffalo, New York, USA Published online: January 2006 DOI: 10.1038/npg.els.0003943 http://www.els.net/WileyCDA/ElsArticle/refId-a0003943.html
Two models of the mammalian PDH organization. (a) Cut-away model of a fully assembled PDH. Lipoic acid dehydrogenase molecules (red) are bound inside the pentagonal dodecahedron core formed by 60 inner domains of Acyl transferase (AT) (green). Pyruvate devarboxylase (yellow) is bound to the AT-core through the inner linkers of AT (blue) (Zhou et al., 2001). (b) A model of the inner core formed by AT. Twelve inner domains of BP (red) replace 12 inner domains of AT and bind with 48 inner domains of AT (blue) to form a 60-mer pentagonal dodecahedron (Hiromasa et al., 2004).Lioubov G Korotchkina, SUNY at Buffalo, Buffalo, New York, USA Published online: January 2006 DOI: 10.1038/npg.els.0003943 http://www.els.net/WileyCDA/ElsArticle/refId-a0003943.html
Pyruvate dehydrogenase is a large multi-enzyme complex organized to provide easy transfer of substrates to each of three different active sites. One cofactor, lipoic acid, is covalently bound to one of the enzymes yet must have access to all three. The active portion of lipoic acid is one a long arm that is in turn covalently linked via an amide bond to a lysine. This combination gives it a long reach. The three different enzymes are:
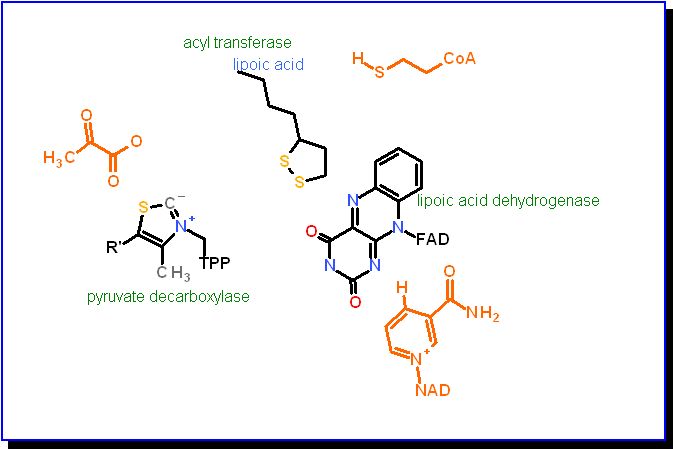 |
The cofactors that are permanent to each active site (TPP, Lipoic acid. FAD) are colored according to atom type. The cosubstrates that come and go with each turn-over event are colored orange. The enzyme names are in green. |
|
The reaction begins with Pyruvate binding to the active site of Pyrivate Decarboxylase enzyme actve site. | |