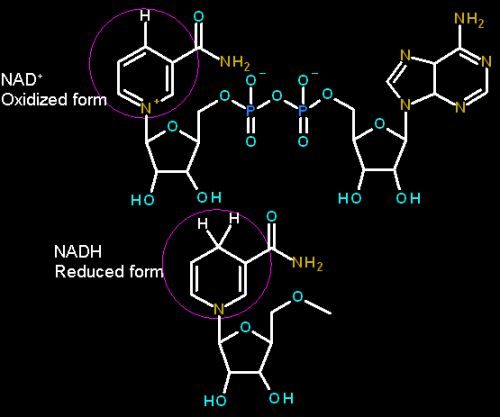
The part that is directly involved in the redox chemistry is circled in magenta
There are two version of this cosubstrate, NAD+ and NADP+. They differ in structure solely in that NADP+ has an additional phosphate bonded to the sugar (on the right in the top structure above).
NAD and NADP have virtually the same role in terms of their chemistry. They are a cosubstrate in redox reactions (not tightly bound to the enzyme but freely diffusible) in which a hydride is transferred to or from the other substrate. They can only transfer two electrons at a time in the form of a hydride (H-)
NAD and NADP have slightly different roles physiologically. typically NADP is associated with reactions that are generally thought as building molecules, while NAD is involved in reactions that are typically thought as tearing down molecules.
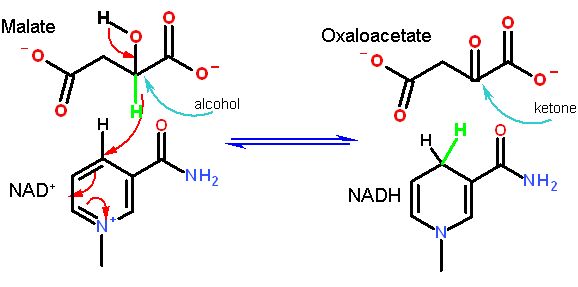
SOME ENZYMES USING THIS COFACTOR
