 |  | |
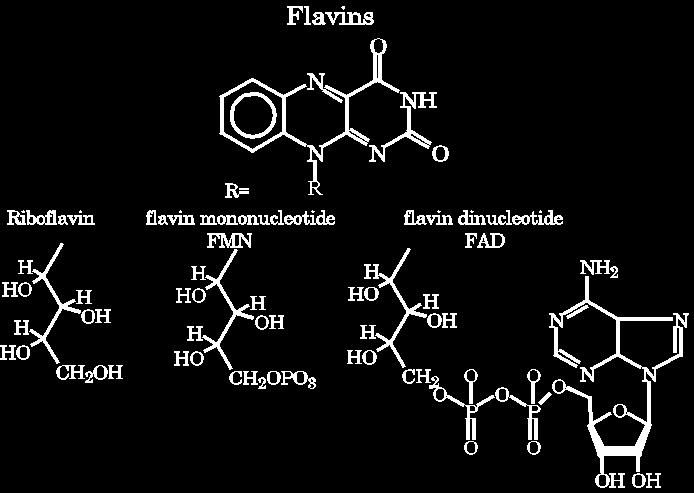
Chemical structures of Rioflavin, flavin mononucleotide (FMN) and flavin adenine dinucleotide FAD
There are three "flavors" of flavins. They active portion of their structures are all the same (at the top of this figure). They differ only in the structures of the "R" group. These R groups are not involved in the chemistry of the flavins. |
3D representation of FAD |
Vitamin B2 Dependence: Humans do not synthesize flavins (the active portion of these cofactors) and therefore require its intake as part of the diet. Riboflavin is one of the (many) B types typically called vitamin B2. You may read much more about this and issues regarding its synthesis and deficiencies here.
|
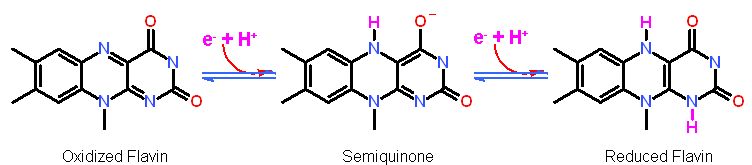
| Flavins are enzyme bound cofactors that are involved in electron transfer. The isoalloxazine ring structure (the active portion) can take up either 1 or 2 electrons with an appropriate number of protons. The cofactors are tightly bound to the enzyme with or without a covalent linkage (depending on the enzyme). The fact that flavins can transfer either one or two electrons makes them ideal "adapters" between those cofactors that MUST transfer two at a time (i.e. NAD+) and those that MUST transfer only one at a time (i.e. hemes and Fe/S clusters). The semiquinone is rarely observed as it is very short lived. The oxidized and fully reduced form are the much more common.
|
 |
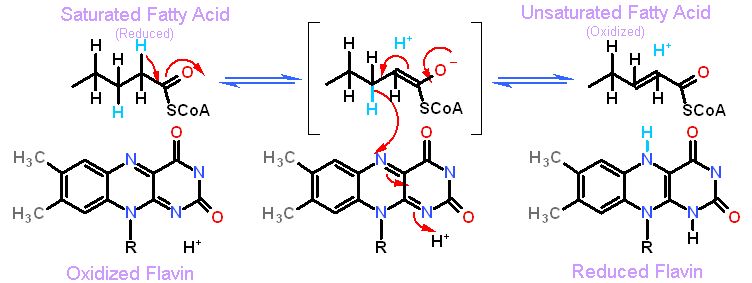
| Flavins can also be directly invovled on chemistry. It is frequently used with reactions that make an unsaturate C=C bond. One example is fatty acid desaturase, shon below.
|
 |
| SOME ENZYMES USING THIS COFACTOR |
| succinate dehydrogenase This is an enzyme that is part of the Krebs cycle that we will learn more about in module 7 |
pyruvate dehydrogenase (key transition enzyme between glycolysis and the Krebs cycle) - more about this enzyme in module 6 |


