|
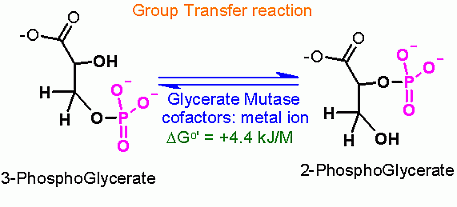
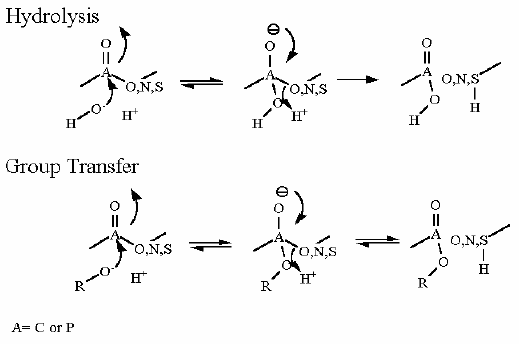
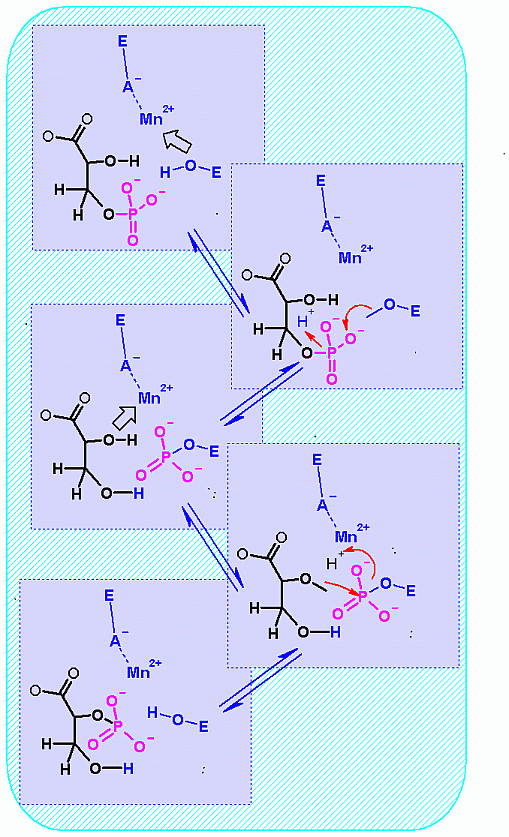
This is a group transfer reaction. This is most easy to visualize when you look at the mechanism of the reaction (see below). The phosphate is transferred from one alcohol to another through a phopshoenzyme intermediate. In effect there are two group transfer reaction... 1. transfer of phosphate to the enzyme and 2. transfer of phosphate from the enzyme back to glycerate.
Why this is NOT an isomerization reaction.... Note that 3-P glycerate and 2-P glycerate are in fact isomers (they share a common chemical composition but one bond is different). Just because they are isomers does not makde this an isomerization reaction. To the enzyme mechanism an isomerization is a a "swapping" of an adjacent alcohol and aldehyde carbons.
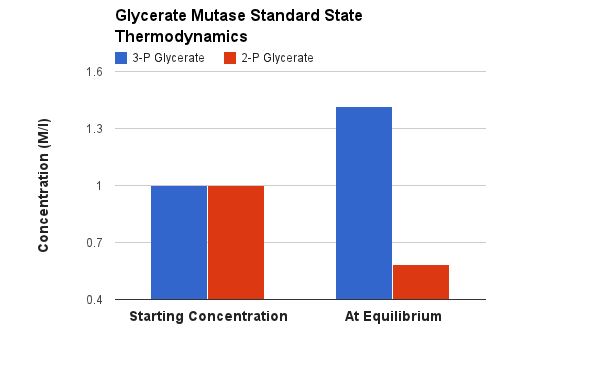
Why is Phosphate moved from C3 to C2 - what good is it? This is justified by the next two reactions. Recall what I said about glycerate kinase - that we can transfer a PO4 to ADP by group transfer if the Standard Free Energy favors it (or is at least close). Right now we are trying to build to one those compounds that has a very high Standard Free Energy of hydolysis for the phosphate group. That high favorable Free Energy will be used to geneate another pair of ATP.
|