|
Enzyme Name |
Triose Phosphate Isomerase | |
Reaction Catalyzed |
Isomerization between Dihydroxyacetone Phosphate (DHAP) and Glyceraldehyde-3-Phosphate (G3P) 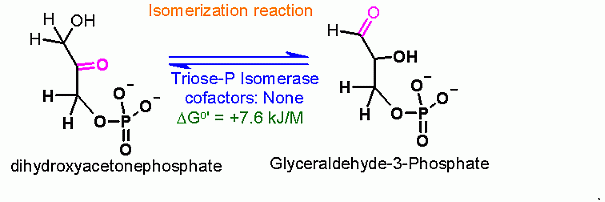 | |
Reaction Type |
Isomerization | |
Pathway Involvement |
Glycolysis AND gluconeogenesis |
|
Cofactors/Cosubstrates |
None | |
|
Rationale |
The pieces from the aldol reaction are not identical, but are related to each other by a simple conversion. Another isomerization! On one of the halves of the aldol reaction (dihydroxyacetone-Phosphate) the ketone is on C2 while on the other it (glyceraldhyde-3-Phosphate) is on C1. How can they be made to look identical? The glyceralhehyde does NOT NEED to do an isomerization. ONLY dihydroxyacetone phosphate (ketone on C2) needs to be isomerized. Afterwards it is identical to the other half (glyceraldehyde-3-phosphate). FOR ALL REACTIONS FROM HERE ON, THERE ARE TWO MOLECULES GOING THROUGH EACH CONVERSION FOR EACH GLUCOSE THAT STARTED.
| |
|
ΔGo' |
+7.6 kJ/M in the direction of glycolysis |
 |
Keq |
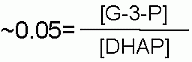 |
|
Comments |
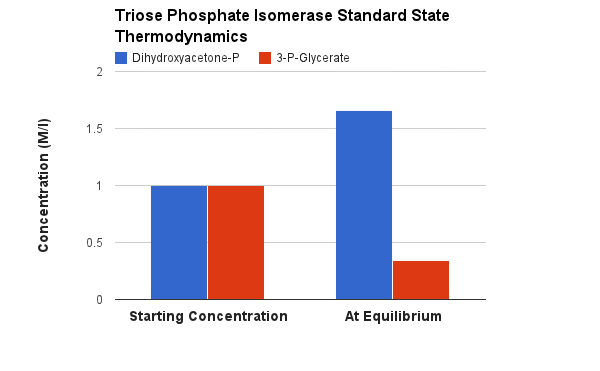
Starting from standard state and allowing the reaction to come to equilibrium the dihydroxyacetone phosphate (DHAP) concentration would end up ~20 times higher than the concentration of glyceraldehyde-3-phosphate. The Standard Free Energy favors dihydroxyacetone phosphate production. This is the classic paradigm enzyme for isomerization reactions. It has been more well studied than almost any other enzyme. Even the protein fold ("pictures") is called the TIM β-barrel. TIM = Triose phosphate IsoMerase. Note the the barrel that is formed as β sheets structures wrap around the center of the protein.
| |
"In cell" Substrate Concentrations* |
||
S1 = |
dihydroxyacetone phosphate | 0.14 mM |
S2 = |
||
P1 = |
Glyceraldehyde-3-Phosphate | 0.019 mM |
P2 = |
||
ΔG for these conditions |
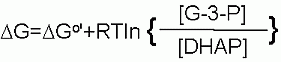 | +2.4 kJ/M |
| Note: Even under the normal conditions the thermodynamics STILL favors the production of dihydrozyacetone phosphate. But it is LESS positive than the standard state equilibrium Here is where the we have to understand that living systems are not static. Meaning nothing stays as is. The system is always changing. Nothing in living system comes to equilibrium - until you die. Everyting depends on that there is enough Glyceraldhyde-3-P to make the NEXT enzyme in the pathway perform its function. |
||
|
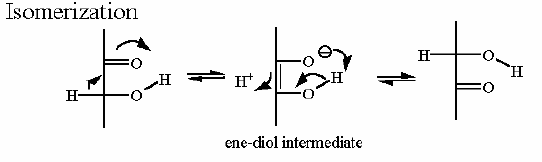
Mechanism for Chemistry |
 | |
Mechanism for Enzyme |
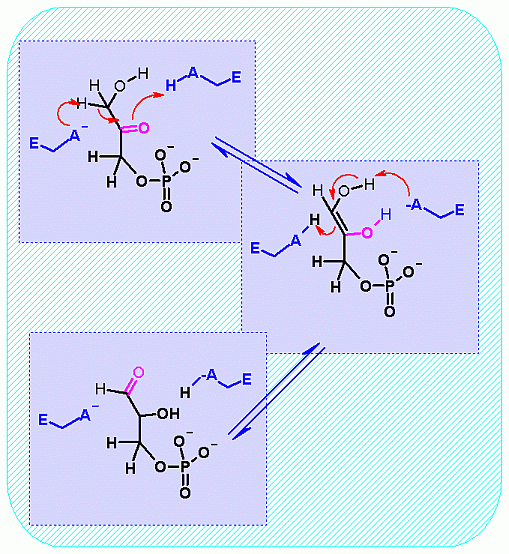
Hover your mouse over a step to reveal a description  Triose Phosphate Isomerase. Animation of the Triose Phosphate Isomerase reaction Blue: represents the enzyme. The E-A- and E-AH represent the crucial enzyme active site amino acids in their basic (deprotonated) and acidic (protonated) states, respectively. "Start" begins an animation of the isomerization reaction. It proceeds through the reaction in the "forward" direction and then "backwards" again. Note how the enzyme is involved. "+" increases speed while "-" decreases the animation speed. You may also step through the reaction using "next" or "previous"
Triose Phosphate Isomerase. Animation of the Triose Phosphate Isomerase reaction Blue: represents the enzyme. The E-A- and E-AH represent the crucial enzyme active site amino acids in their basic (deprotonated) and acidic (protonated) states, respectively. "Start" begins an animation of the isomerization reaction. It proceeds through the reaction in the "forward" direction and then "backwards" again. Note how the enzyme is involved. "+" increases speed while "-" decreases the animation speed. You may also step through the reaction using "next" or "previous"
Notice the "push - pull" of the base and acid to initiate the isomerzation to the "ene-diol" intermediate - and then they "reverse" their function to complete the reaction This reaction can obviously go both ways and indeed the Standard Free Energy favors the DHAP substrate. Compare the animated reaction to the "arrow pushing" scheme at the right. See if you can correlate the electron movement in the animation to the arrows in the static picture above. | |
|
Picture of Enzyme with substrate |
|
|
|
Click on an atom to diplay identity here | |
|
|