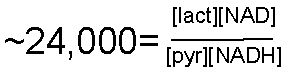|
Enzyme Name |
Lactate Dehydrogenase |
||
|
|
|||
|
Reaction Catalyzed |
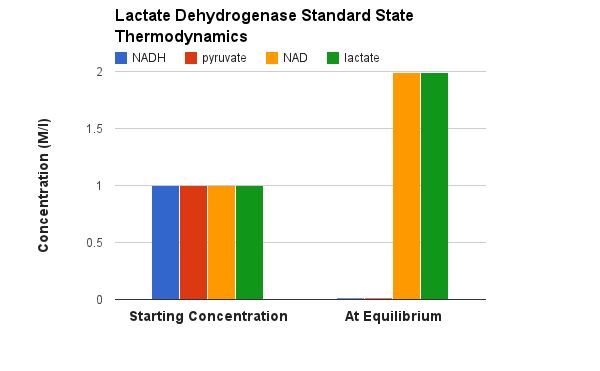
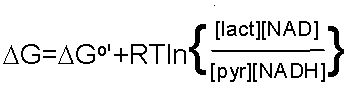
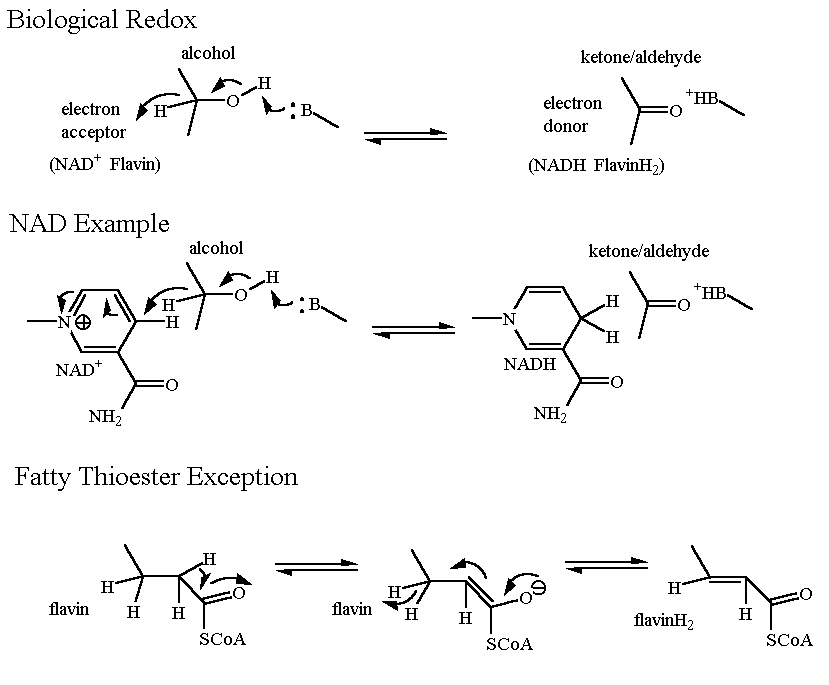
Oxidation/Reduction Pyruvate is reduced to Lactate OR Lactate is oxidized to Pyruvate
|
||
|
Reaction Type |
Redox Reaction |
||
|
Pathway Involvement |
As pointed out it is not officially part of either Glycolysis or Gluconeogenesis The reaction is necessary however to support glycolysis under anaerobic conditions. As a side note... in bacteria the lactate is excreted into the media - if the media happens to be milk then the lactate lowers the pH of the milk - the milk proteins denature and coagulate, it gels and we eat it because it tastes good - it is called Yogurt. In mammals muscle cells can do this - the lactate is excreted into the blood stream. it is taken up by the liver (mostly) and regenerated into glucose (via gluconeogenesis) Red blood cells also use this process. RBCs do not have much of a need for metabolism and do not have mitochondria (or a nucleus for that matter). They depend on lactate fermentation for all their (limited) energy needs. |
||
|
Cofactors/Cosubstrates |
NADH/NAD+ is a cosubstrate/coproduct |
||