|
Why is another phosphate transferred to Fructose?
A Second ATP dependent group transfer. After the isomerization can we do a group transfer at C1? Why or could we not transfer a phosphate to C1 of glucose? Why do we need to do transfer another phosphate at all?
Think about the two pieces after an aldol cleavage. If only one phosphate were on fructose, Would both fragments be charged? If both fragments are not charged, then what would happen to the uncharged one?
Furthermore, both of these phosphates will ultimately be in a very high energy yield functional group. They will both be in an ENol of phosphoenol pyruvate and subsequetly transferred back to make ATP again.
Complicated kinetics
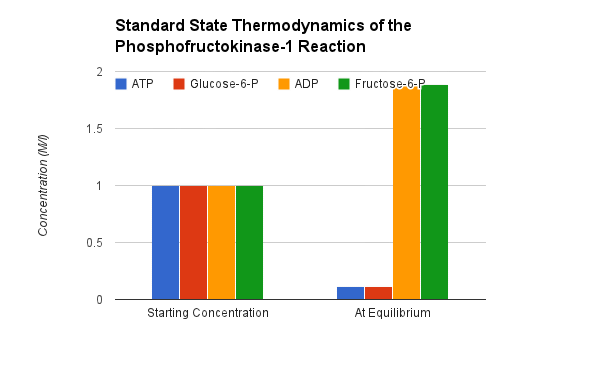
As one might guess, It would be somewhat problematic to have both glycolysis and gluconeogenesis pathways operating at full capacity together. Rather there must be a coordinated way to ensure that one pathway is "crippled" while the other is operating well. How this occurs will be explained ad nauseum in module 8.
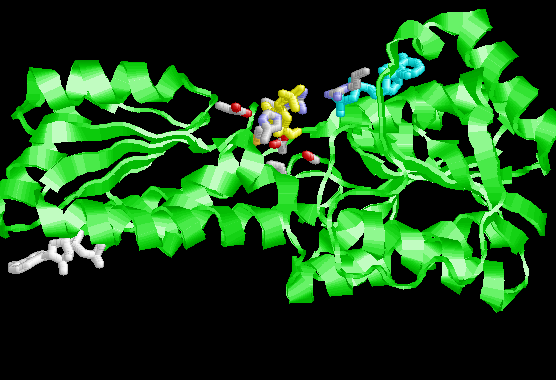
There are four identical subunits in the active enzyme.
This is an allosterically (literally means another site) regulated enzyme and is one of the key regulation points between glycolysis and gluconeogenesis. MUCH more about this in module 8. For now just bear in mind that the kinetic properties of this enzyme can be greatly altered by compounds binding to a site that is well removed from the active in the protein structure.
This enzyme is PFK-1. There is another phosphofructokinase (PFK-2) that is not involved in the metabolic pathway. This a a separate enzyme that transfers a phosphate to C2 of Fructose-6-phosphate. to produce Fructose-2,6-bisphosphate. this compound is not metabolized, but rather serves ONLY a regulatory function. We will learn much more about this enzyme and the role of fructose-2,6-bisphosphate in Module 8.
This is an allosterically (literally means another site) regulated enzyme. One of the key regulation points between glycolysis and gluconeogenesis. MUCH more about this in moule 8. For now just bear in mind that the kinetic properties of this enzyme can be greatly altered by compounds binding to a site that is well removed from the active in the protein structure.
| 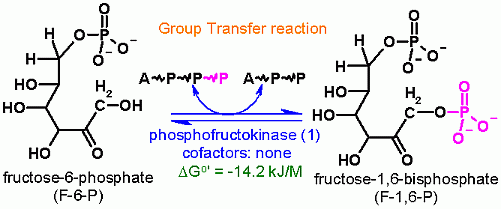

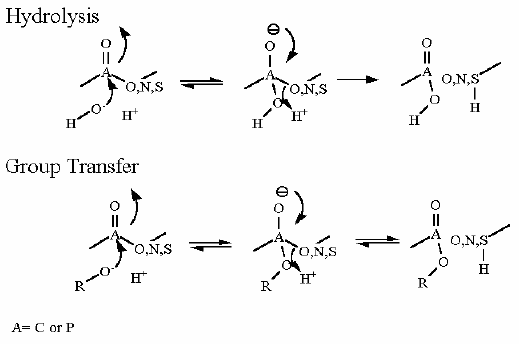
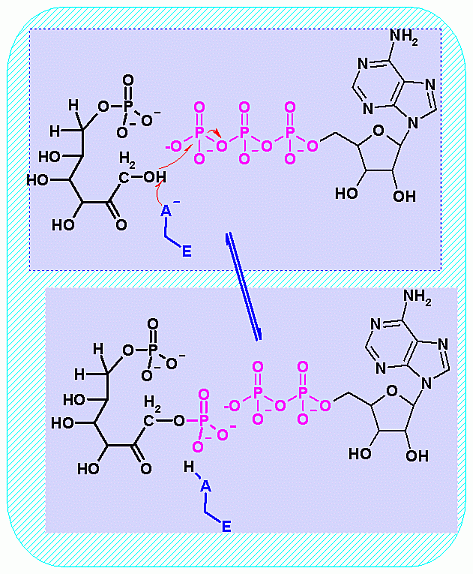
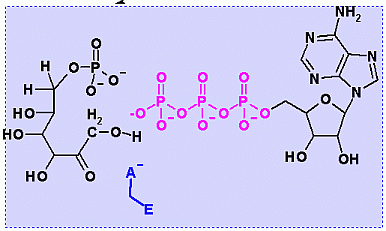 Phosphofructokinase PFK-1. Animation of the PFK-1 reaction Blue: represents the enzyme. The EA- represent the crucial enzyme active site amino acids in their basic (deprotonated) . "Start" begins an animation of the group transfer reaction. It proceeds through the reaction in the "forward" direction and then "backwards" again. Note how the enzyme is involved. "+" increases speed while "-" decreases the animation speed. You may also step through the reaction using "next" or "previous"
Phosphofructokinase PFK-1. Animation of the PFK-1 reaction Blue: represents the enzyme. The EA- represent the crucial enzyme active site amino acids in their basic (deprotonated) . "Start" begins an animation of the group transfer reaction. It proceeds through the reaction in the "forward" direction and then "backwards" again. Note how the enzyme is involved. "+" increases speed while "-" decreases the animation speed. You may also step through the reaction using "next" or "previous"