A Redox reaction involves changing the oxidation state of a Carbon - with electrons going to a different molecule (not just being rearranged within it)
Examples of Oxidations (electrons leave the molecule and go to another)- Changing an alcohol to ketone/aldehyde
- Changing an aldehyde to an acid
- Changing a C-C (Carbon single bond with only C or H attached) to a C=C (Carbon double bond)
- Changing an ketone/aldehyde to an alcohol
- Changing an acid to an aldehyde
- Changing a C=C (Carbon double bond) to a C-C (Carbon single bond no new bonds involve O/N or S)
Electron Carriers
As pointed out earlier, electrons are never free in solution - they are always part of a larger molecule. The electrons for these reactions must either go somewhere or come from somewhere. Further, we will find out later that electrons need to get physically moved from one cell compartment (cytosol) to another (mitochondria). We need another universal adapter to interface between a variety of reactions and the mitochondrial systems. That role generally falls to Nicotine Adenine Dinucleotide (NAD+ - I'll explain the "+" part better later) While NAD+ is the commons carrier of electrons from one place to another, it is not always directly involved in the chemistry, buther there may be other mediators along the way.
- In oxidizing a compound the electrons usually go directly to NAD+ (to produce NADH) or a Flavin (to produce a reduced flavin)
- In reducing a compound the electron usually comes from NADH (producing NAD+) or a reduced flavins (producing a flavin) *
Biological Oxidation Reduction (REDOX) Reaction
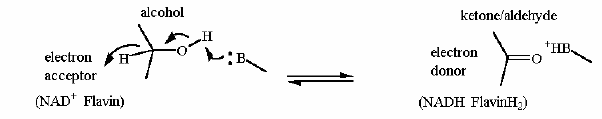
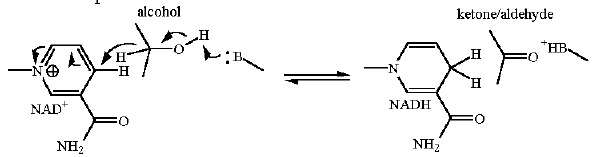
Going left to right... the alcohol carbon is oxidized to a ketone. The hydrogen AND the pair of electrons that is bonding that the H to the alcohol carbon is moved to NAD+ all as one unit producing NADH. Flavin dependent desaturation of a fatty acid
The flavin cofactor can only catalyze this reaction between the α and β carbons of a fatty acid if it is joined to Coenzyme A through a thioester
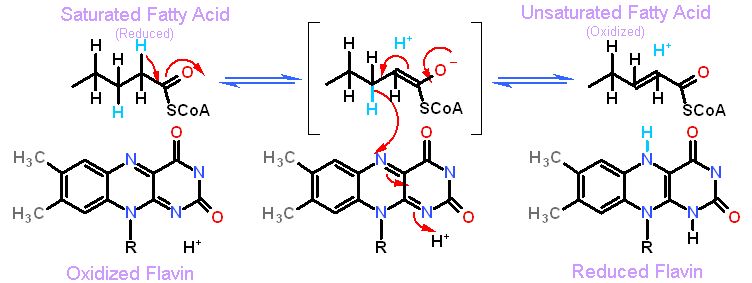
A couple of rules
- NAD+ is generally the cosubstrate (a cosubstrate comes into and leaves the active site of the enzyme with every reaction cycle just as the primary substrate.
- Oxidation an aldehyde to an acid usually uses NAD+ as a cosubstrate and frequently involves synthesis of ATP.
- The reduction of an organic acid to an aldehyde REQUIRES ATP hydrolysis as well as NADH to provide the electrons.
- Converting an saturated carbon (C-C) to an unsaturated (C=C) generally utilizes flavin as a cofactor (a cofactor can be considered part of the enzyme as it does not leave the active site like a substrate)
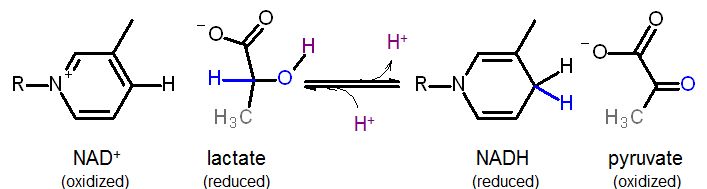 Example from the end of the glycolysis pathway showing the reduction of pyruvate by NADH to produce lactate and NAD+
Example from the end of the glycolysis pathway showing the reduction of pyruvate by NADH to produce lactate and NAD+ 