 |
 |
 |
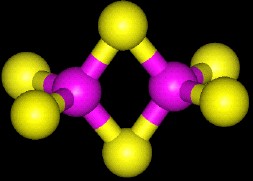
| 3D Structure of a 2Fe-2S cluster. |
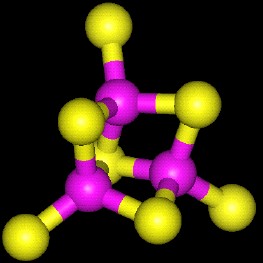
3D Structure of a 3Fe-4S cluster. |
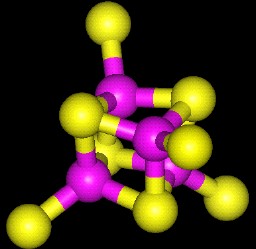
3D Structure of a 4Fe-4S cluster. |
| The centers are composed of three different types of atoms: Iron, inorganic sulfide (S2-), and Cysteine sulfur. In the pictures above, Iron atoms are purple and Sulfur yellow. Inorganic sulfide are bonded to two or more iron atoms each. The entire complex is covalently held to the protein polypeptide by the sulfurs of cystiene residues (at the periphery of the pictures and are the sulfur atoms that stick out of the cluster). |
| The most common function for these clusters is simple electron transfer; one electron at a time regardless of the number of irons. All of the clusters that fall into this category have two physiologically relevant redox states available. Taking into account only the inorganic sulfide (as 2-) and the Fe (as either 3+ or 2+) the formal states available to t 2Fe-2S cluster are +2 (oxidized; two Fe3+) and +1 (reduced; one Fe3+ and one Fe2+) |
 |
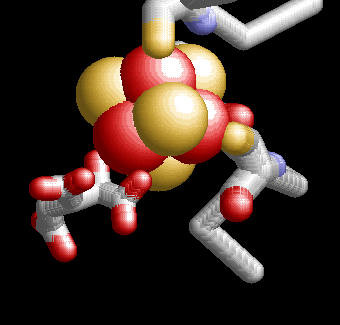
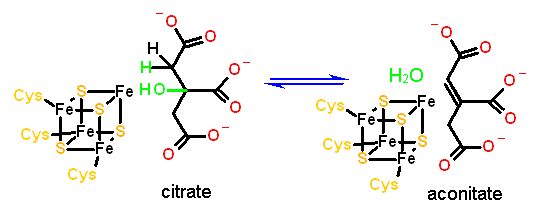
A small number of enzymes have an Iron sulfur cluster that is required to catalyze an elimination reaction. The best example of this type of enzyme is aconitase (part of the Krebs cycle that we will look at in module 7). In an elimination reaction the cluster does NOT change redox state but rather acts as an electrophile to catalyze the deprotonation of water to HO-. The structure of the active center of aconitase cluster is shown below. It has the SAME cubic structure of a 4Fe-4S cluster (as above). However, one of the irons is NOT coordinated to a polypeptide cysteine but rather left available to coordinate to substrate (In this case isocitrate). There are two oxygens of citrate in contact with the iron. one is from the organic acid (front right), while the other is the hydroxyl group of the alcohol carbon (back left). This latter is the hydroxyl group removed from citrate. |
 |  |
| SOME ENZYMES USING THIS COFACTOR AS A REDOX COMPONENT |
| NADH- Coenzymes Q reductase Otherwise known as Complex I - part of the oxidative phosphorylation electron transport system... actually has several clusters |
| Coenzyme Q Cytochrome c reductase Otherwise know as Complex III -part of the oxidative phosphoylation electron transport system... actually has several clusters |





