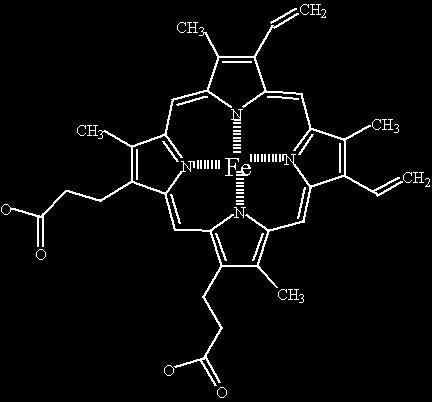
There are several variants, but these are not shown
Representation of electron transfer with hemes like in cytochrome c. The ellipse represents the ring structure, the red circle the central iron atom. Simple Transfer... one in - one out. The iron cycle between the +2 and +3 redox states.
Representation of reversible oxygen binding like in hemoglobin. The ellipse represents the ring structure, the red circle the central iron atom. Simple reversible binding of molecular oxygen. Note the heme must stay in the +2 state throughout.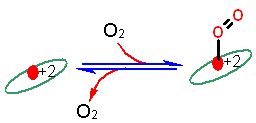
Simplified Representation of a P450 catalytic cycle. The ellipse represents the ring structure, the red circle the central iron atom. The six membered ring is not to represent cyclohexane in this case, but rather a nondescript substrate. Many P450 enzymes can act on a wide range of substances. The catalytic cycle is very complex and is still being studied intensively. some of the steps are "poorly" shown here.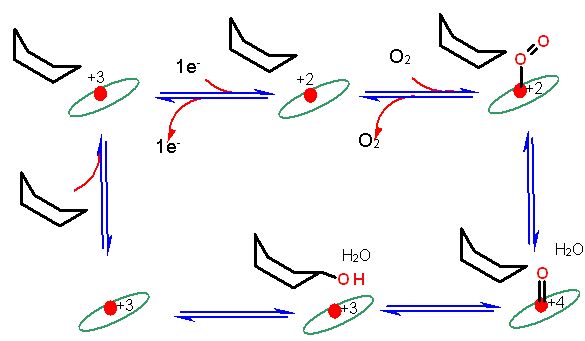
Note: the P450 moniker is a throwback to the beginnings of biochemistry. Before we knew anything about how this enzyme worked or what it did, it was noted that this type of protein exhibited a unique spectrum in with visible light that had a peak absorption at 450nm.
