

There are four protein complexes directly involved in electron transport from NADH to O2 called imaginatively: Complex I, Complex II, Complex III, and ComplexIV. Each of these major protein complexes containing several subunits in a single functional complex are embedded in the inner mitochondrial membrane.... Actually spanning the membrane such that some sticks into the inner matrix while another portion sticks into the inter membrane space (IMS). There is also one small protein: cytochrome c; and one free organic cofactor, Coenzyme Q (Ubiquinone) Each has a specific role.
First the order of electron transfer
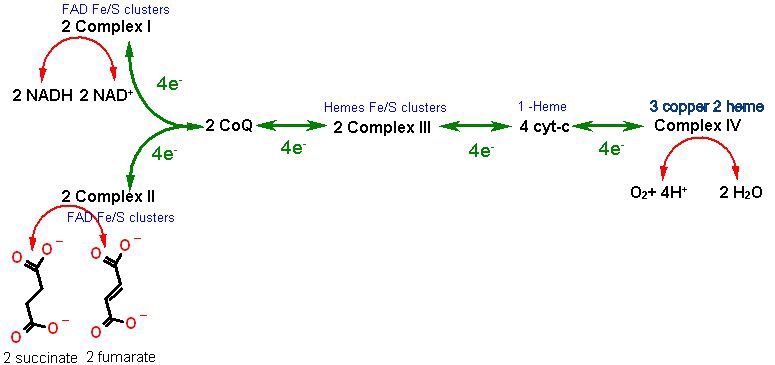
| Protein / Complex/ Cofactor | Function | Cofactors | H+ Transport |
| Complex I | NADH/CoQ oxidoreductase Oxidation of NADH to NAD+ on the inner matrix side transfer electrons to Coenzyme Q (dissolved in the inner membrane) | FAD, Fe/S clusters | YES |
| Complex II | Succinate Dehydrogenase (part of the Krebs cycle) Oxidation of succinate to fumarate on the inner matrix side transfer electrons to Coenzyme Q | Flavin, Fe/S clusters | NO |
| Coenzyme Q | Oxidation of Complex I AND complex II (on the IMS side) transfers electrons to Complex III | NO | |
| Complex III | CoQ/cytc oxidoreductase Oxidation of Coenzyme Q and transfers electrons to cytochrome c on the IMS side | Coenzyme Q, Fe/S clusters, Hemes | YES |
| cytochrome c | Oxidation of Complex III transfer electrons to Complex IV on the IMS side | 1 c-type Heme | NO |
| Complex IV | Cytochrome c oxidase Oxidation of cytochrome c IMS side and reduction of O2 to H2O on the inner matrix side | 2 Hemes, 2 copper ion | YES |
The end results of this electron transport system.... So far
So far glucose has been oxidized from the carbohydrate to CO2. CO2 eventually gets exhaled. All the electrons from the oxidation were initially transferred to NAD+ and one FAD. But these cofactors cannot stay in the reduced state because of their limited supply. Therefore we used the electron transport system to tranfer the electrons from NADH and reduced FAD all the way to O2 to produce water. It is essential that oxygen is reduced all at once to avoid producing dangerous intermediately reducued oxygen species (superoxide, hydrogen peroxide and hydroxyl radical). O2, of course is inhaled from the environment.
The end result...so far... the m&m's that were initially consumed have been conveted to CO2 and sent packing in your outgoing breath. The O2 contained in your incoming breath has been converted to water.
While the chemistry is complete, there is a mjor pience mssing. There is no connection to synthesis of ATP beyond the 4 (equivalents) that were made in glycolysis and the citric acid cycle. I made a big deal about there being enough energy in each pair of electrons to make a bunch of ATP... where is it?