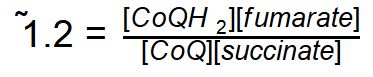|
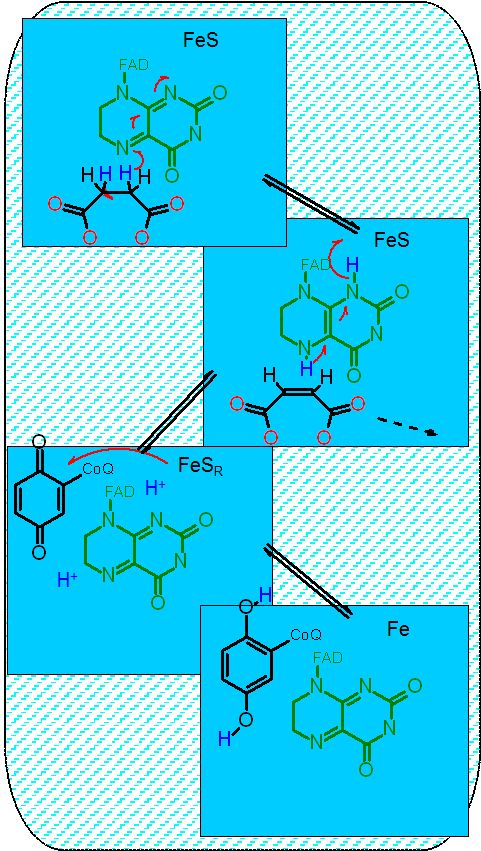
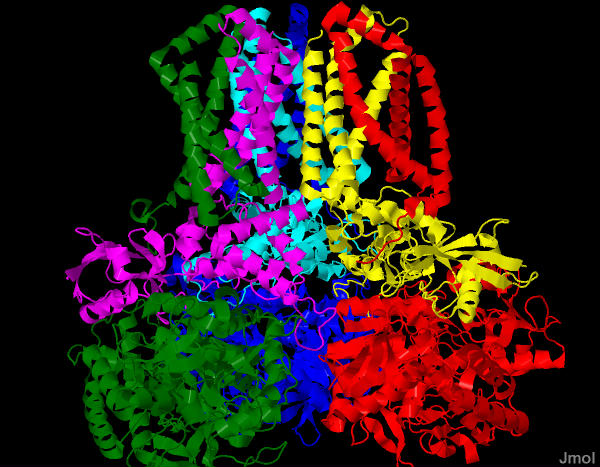
This is a membrane bound enzyme - embedded in the inner mitochondrial membrane. A large part of it sticks in to the inner matrix - this is where the succinate chemistry occurs. But also much of the protein is buried completely in the membrane (SEE PICTURES) allowing it to interact with the very hydrophobic coenzyme Q.
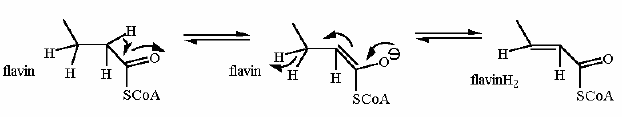
So we are now back to four carbons, 2 of them organic acids. Seems like we are pretty close- BUT there is one essential problem. Remember, that the first step is an aldol reaction to add acetate. That requires a ketone/aldehyde. All we have are 2 organic acids and two methylene carbons. We need to covert one of those two methylene carbons into a ketone. This takes three step -of which this is the first.
This reaction generates unsaturated carbons next to an organic acid using a flavin cofactor. This is a common reaction especially in metabolism of fatty acids. We will not specifically cover this in this course... but suffice it to say that long chain fatty acids are put together from acetate (acetyl-CoA actually) and then a series of three reactions just like this one (and the next two from the citric acid cycle) are combined to complete the process.
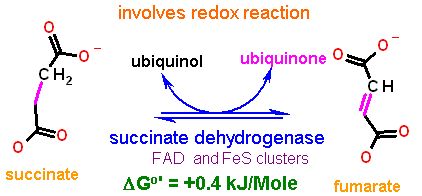
This is a complex looking reaction as it takes not only the flavin (FAD), but alos a series of iron sulfur cluster and a heme. Note also that Coenyzme Q is listed as the final recipient of electrons.
We will find in pater pages of this chapter that this enzyme has another name as well - That of Complex II. Ultimately the electrons from succinate will end up being passed down to oxygen via the electron transport chain.
|