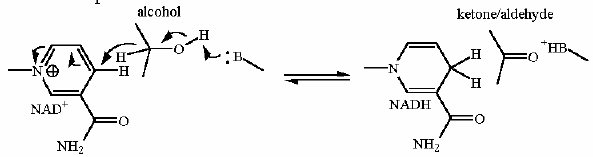|
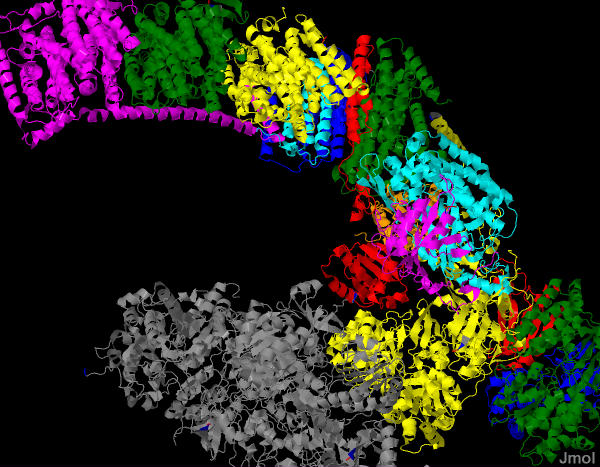
The official database for keeping protein structures also as a Molecule of the month page. This one addresses complex I or NADH Dehydrogenase. The complete structure has recently (2011) been solved. It is an extremely complex protein containing many subunits and cofactors. There are two separate - but - coupled reactions: electron transfer, H+ pumping from the inner matric to the intermembrane space.
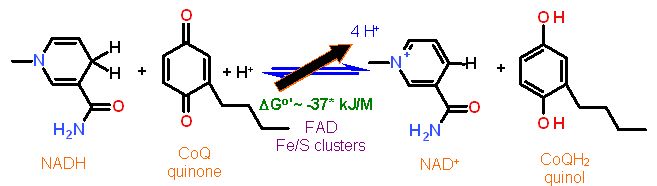
The main mechanism for NADH Dehydrogenase is electron transfer from NADH to CoQ. This is a redox reaction. Our ultimate goal is to make ATP which is a condensation (reverse of hydrolysis). These are not compatible reaction types. You cannot use one directly with the other. Some kind of 'adapter' must be used. kind of like trying to fit a three prong plug into a 2 prong outlet... you need an adapter. The generation of the H+ gradient is the first step in this 'Rube Goldberg' process. Later another type of protein will turn the gradient into useful energy yo make ATP.
This complex pumps about 4 H+ from the inner matrix to the inter membrane space for every pair of electrons transferred.
The are four similar subunits in the protein complex that are embedded (and span) in the membrane. These are thought to be the portions that are responsible for H+ translocation into the intermembrane space. There is another portion of the complex that contains all of the redox cofactors and this projects into the inner matrix.
Looking at the pictures below, it is plain to see that the portions of the protein that are responsible for electron transfer and those that are responsible for the translocation of H+ across the membrane are separate. It remains unclear at this time how the four subunits that move H+ are controlled by the changes in redox state of the cofactors or even how the four similar subunits work together.
|