|
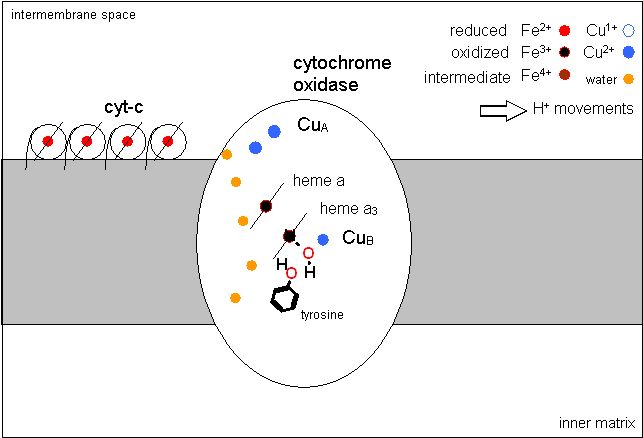
The official database for keeping protein structures also as a Molecule of the month page. This one addresses complex IV or cytochrome c oxidase. It is an complex protein containing many subunits and cofactors. This complex also pumps H+, and is responsible for the 4 electron reduction of O2 to H2O.
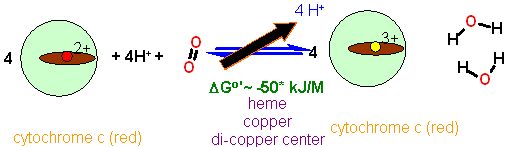
The main mechanism for complex IV is electron transfer from cytochrome c to molecular oxygen. This is a redox reaction. Our ultimate goal is to make ATP which is a condensation (reverse of hydrolysis). These are not compatible reaction types. You cannot use one directly with the other. Some kind of 'adapter' must be used. The generation of the H+ gradient is the first step in this 'Rub Goldberg' process. Later another type of protein will turn the gradient into useful energy yo make ATP.
This complex pumps about 4 H+ from the inner matrix to the inter membrane space for every pair of electrons transferred. This H+ translocation is associated with the electron transfer itself and not the oxygen reduction. The transfer of electrons from the CuA site makes (relatively speaking) a region of positive charge. This has the effect of moving the positive charged H+ away from this spot... into the intermembrane space. As the site is reduced again another H+ comes back in. This happens four times for each oxygen reduction. As Oxygen reduction requires four electrons... four H+ are translocated to the intermembrane space for each oxygen reduced.
This is the final stop for the electrons. They are stuffed into O2 with the formation of water - which we eliminate eventually.
There is an unusual "water channel" through the middle of this protein. This water channel is essential for H+ pumping. Arg (38) Met (390) and Val (386). The Arg forms and essential part of the proton translocation chain in the channel. The other two are essential in another way. There is a water very near the sulfur of the methionine. It has been demonsrated that IF the methionine is replaced by tryptophan (a much larger group) THEN this water is not present. The effect is that the water channel is blocked and H+ pumping does not occur - even though Oxygen reduction occurs at a normal rate.
|