
The idea in this group transfer reaction is to have the -OH group on the C6 alcohol carbon of glucose to "attack" the terminal phosphate of ATP.
-OH is not a good attacking agent for a group transfer reaction- it would be much better if it were unprotonated -O- instead.
The results of the previous steps are shown.
Not shown: as the pH of the surrounding solution is higher than the pK for Aspartic Acid, the proton will likely dissociate and thus regenerate the base for of Aspartate again so that the enzyme can continue to function.

| Reaction | Rationale | Thermodynamics | Mechanism | Pictures | JMOL |
|
Reaction catalyzed |
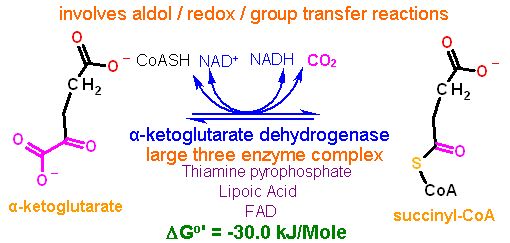
|
|
The enzyme α-ketoglutarate dehydrogenase catalyzes this conversion. This conversion is quite complex and requires FIVE vitamin derived cofactors to complete. This is very similar to that for the pyruvate dehydrogenase enzyme complex.. in fact the mechanism and overall enzyme structures are very similar... the only difference - this one catalyses the decarboxylation of α-ketoglutarate instead of pyruvate. | |
| Reaction Types |
|
| Cofactors/Cosubstrates |
|
| Pathway Involvement | Citric Acid Cycle (also amino acid synthesis - put an amine on the ketone and it is Glutamate) |