 Pyruvate Kinase Information
Pyruvate Kinase Information Pyruvate Kinase Information
Pyruvate Kinase Information
|
Enzyme Name |
Pyruvate Kinase |
|
|
|
||
|
Reaction Catalyzed |
Transfer of phosphate group from phosphenolpyruvate to ADP to make ATP and pyruvate
|
|
|
Reaction Type |
Group Transfer Reaction |
|
|
Rationale |
Elmination Reaction. The phosphate has already been placed on C2
of glycerate from the previous enzyme. The Enol functional group. The significance of the Enol group
In phosphoENOLpyruvate the enol does not terminate with -H but
with -PO3 like compound "B" Thermodynamically speaking hydrolysis of phosphate from
phosphoenolpyruvate has a very high favorable Standard Free Energy
because two things happen... we get the hydrolysis AND then the enol
converts mostly to the much more favorable ketone. How much energy is in the Ketone <-> enol conversion. The ΔGo' as written is +45 kJ/M which meand that the ketone form is favored by a factor of ~65,000,000 over that of the enol form in pyruvate. |
|
|
Pathway Involvement |
Glycolysis ONLY |
The favorable thermodynamics of the enol -> ketone tautomerization virtually precludes this reaction from running in the gluconeogenesis direction. Rather TWO enzymes that EACH use an ATP (or equivalent in GTP) are required to get from pyruvate to PEP in the gluconeogenesis direction
|
|
Cofactors/Cosubstrates |
None |
|
|
|
||
|
DGo' |
-31.7 kJ/M |
Starting from standard state and allowing the reaction to come to equilibrium the Pyruvate and ATP concentration would end up ~20,000 times higher than the product of the concetrations of ADP and PEP. The Standard Free Energy strongly favors ATP production. |
|
Keq |
 |
|
|
Comments |
Note: the Standard Free Energy grealy favors production of ATP.
This is the last of the ATPs generated in the glycolysis pathway.
|
|
|
"In cell" Substrate Concentrations* |
||
|
S1 = |
0.023 mM | 0.031 mM |
|
S2 = |
ADP |
0.14 mM |
|
P1 = |
Pyruvate | 0.0.051 mM |
|
P2 = |
ATP |
1.85 mM |
|
DG for these conditions |
 |
|
|
-23.0 kJ/M |
||
|
|
||
|
Mechanism for Chemistry |
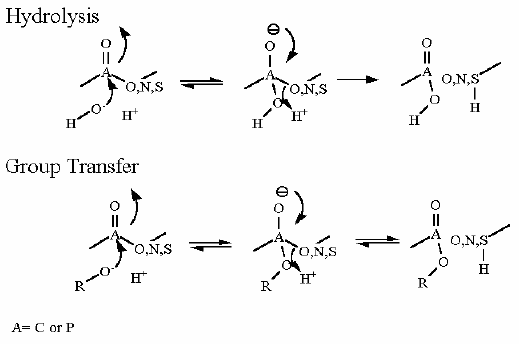 |
|
|
Mechanism for Enzyme |
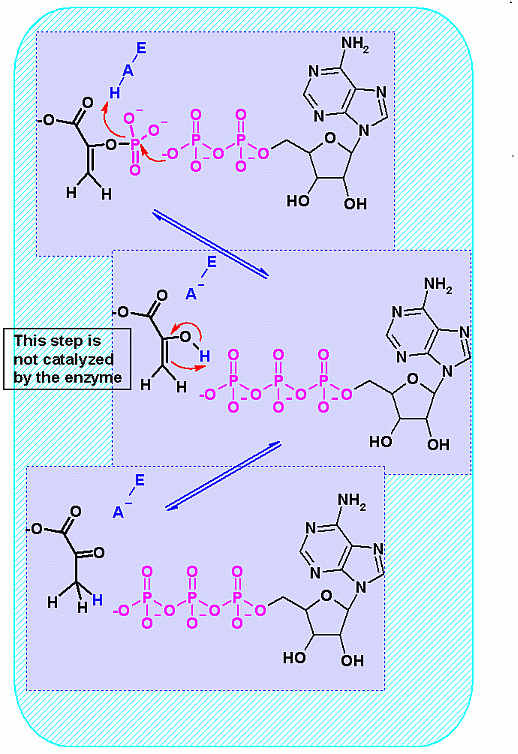
|
|
|
Picture of Enzyme with substrate |
|
|
|
|
|
|
|
Pyruvate KinaseCHIME representation |
|
*= These are concentrations obtained for one set of conditions. These will change as physiology and activity change.