 Pyruvate Carboxylase Information
Pyruvate Carboxylase Information Pyruvate Carboxylase Information
Pyruvate Carboxylase Information
|
Enzyme Name |
Pyruvate Carboxylase |
|
|
|
||
|
Reaction Catalyzed |
Addition of CO2 to C3 of pyruvate via an Aldol Reaction
|
|
|
Reaction Type |
Pyruvate adds "C" via an Aldol Reaction. There are two group transfer reactions to prepare CO2 for this process |
|
|
Rationale |
This is the first of two required enzyme to bypass pyruvate kinase. This one puts another carboxyl group on to pyruvate... so that the next one, phosphoenolpyruvate carboxykinase, can use the Free Energy of decarboxylation to drive the addition of phosphate to the "enol" group. Carbon dioxide cannot be used as CO2 in mammalian systems for addition of a carbon by an Aldol reaction. First and foremost this is becasue we go to great lengths to keep our CO2 concentration low. There are two mechanisms:
The first two steps in this overall reaction are solely to prepare and capture HCO3- so that it is amenable to an aldol reaction with pyruvate. For chemical reasons (see comments below) bicabonate is a very poor substrate for an aldol reaction. The first preparation step is to "activate" HC03- in a group transfer reaction with ATP. This generates the first intermediate, a carboxyl-phosphoanhydride. (notice the similarities of this to the phosphoanhydrides of ATP) and the first product, ADP. The second capture step is to transfer the activated carbon dioxide to Biotin a cofactor that is covalently bound to the enzyme to generate carboxybiotin and phsophate. Carboxybiotin is kinetically stable and can now wait until pyruvate enters the active site. The Aldol reaction between pyruvate and carboxybiotin generates the final product oxaloacetate as well as biotin (so that the enzyme can go through another cycle). |
|
|
Pathway Involvement |
Gluconeogenesis ONLY |
This enzyme is the first of two that are required to bypass the pyruvate kinase reaction of glycolysis. Note that pyruvate kinase has a ΔGo' that so favors glycolysis that it is unlikely that is could be driven backwards. Therefore two ATP (or ATP equivalent) dependent reactions: 1. pyruvate carboxylase 2. PEP carboxykinase, are required to get from pyruvate to phosphoenolpyruvate. |
|
Cofactors/Cosubstrates |
Biotin is required and is covalently bound to the enzyme |
|
|
|
||
|
DGo' |
+3.1 kJ/M |
Starting from standard state and allowing the reaction to come to equilibrium the oxaloacetate concentration would end up ~3 times lower than the concetration of pyruvate. The Standard Free Energy slightly favors pyruvate production. |
|
Keq |
 |
|
|
Comments |
In terms that a chemist would use... activation means adding a good "leaving group". If bicarbonate first attacks ATP... then the "leaving group" is ADP. A very stable compound and is an excellent leaving group. and the end result is that mixed anhydride. The next step is to have biotin attack the mixed anhydride where phosphate is the leaving group (phosphate is also very stable as is) the last step is to have pyruvate do the aldo reaction with carboxybiotin.. now biotin is the leaving group as CO2 is added. biotin is also a great leaving group.. What makes a "good" leaving group? a compound that is very stable as is... as it "leaves" the reactants. In these cases ADP, PO4 and biotin all make greta leaving groups simply because they are compounds that are stable with all the charges etc at pH 7 JUST qas they leave the reaction.... n this regard HO- is not a grood leaving group because it would MUCH rather be protonated and neutral at pH 7. |
|
|
|
||
|
Mechanism for Chemistry |
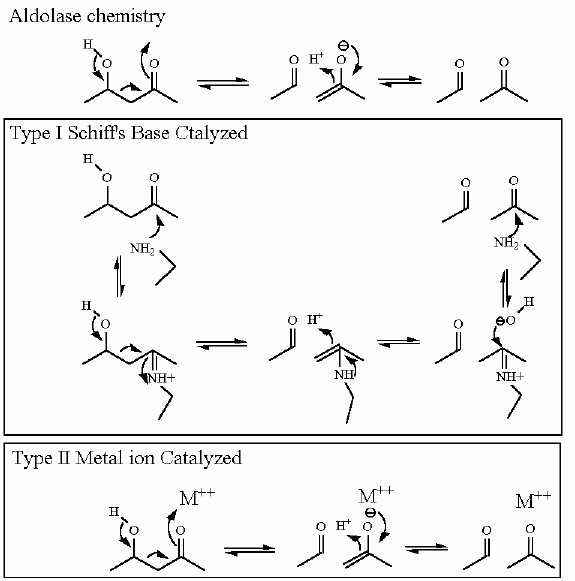 |
|
|
Mechanism for Enzyme |

|
|
|
Picture of Enzyme with substrate |
|
|
|
|
|
|
|
Phosphofructokinase
PFK-1 CHIME representation |
|
*= These are concentrations obtained for one set of conditions. These will change as physiology and activity change.