 Phosphoenolpyruvate Carboxykinase Information
Phosphoenolpyruvate Carboxykinase Information Phosphoenolpyruvate Carboxykinase Information
Phosphoenolpyruvate Carboxykinase Information
|
Enzyme Name |
Phosphoenolpyruvate Carboxykinase |
|
|
|
||
|
Reaction Catalyzed |
Addition of phosphate to pyruvate with comcomitant aldol cleavage of CO2 from Oxaloacetate
|
|
|
Reaction Type |
Concomitant Group Transfer and Aldol Reactions |
|
|
Rationale |
This is the second half of the two reactions that are required to bypass the pyruvate kinase reaction of glycolysis. This one takes the smae carboxyl group that was just put onto pyruvate by the pyruvate carbolylase enzyme. This uses the Free Energy of generating a CO2 from and Aldol cleavage to drive the "enol" formation and then the the "enol" attacks GTP for a group transfer reaction. while the ΔGo' is not very far from 0, this reaction is virtually irreversible toward product phosphoenolpyruvate. The reason is that CO2 concentrations are very low in the tissues. We go to great lengths to keep our CO2 concentration low. There are two mechanisms:
A metal ion help the aldol reaction get started by providing a strong positive charge to start the electron pulling.
|
|
|
Pathway Involvement |
Gluconeogenesis ONLY |
|
|
Cofactors/Cosubstrates |
a metal ion usually Mn2+ is
required as a cofactor |
|
|
|
||
|
DGo' |
-2.2 kJ/M |
Starting from standard state and allowing the reaction to come to equilibrium the PEP concentration would end up ~2 times higher than the concetration of oxaloacetate. The Standard Free Energy slightly favors PEP production. |
|
Keq |
 |
|
|
Comments |
|
|
|
|
||
|
Mechanism for Chemistry |
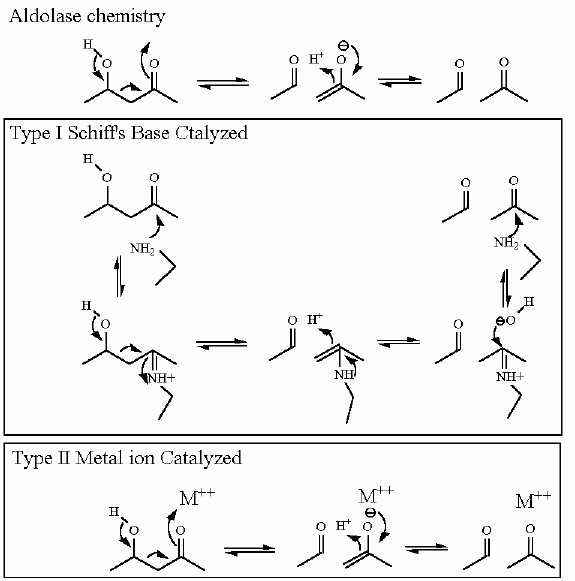 |
|
|
Mechanism for Enzyme |
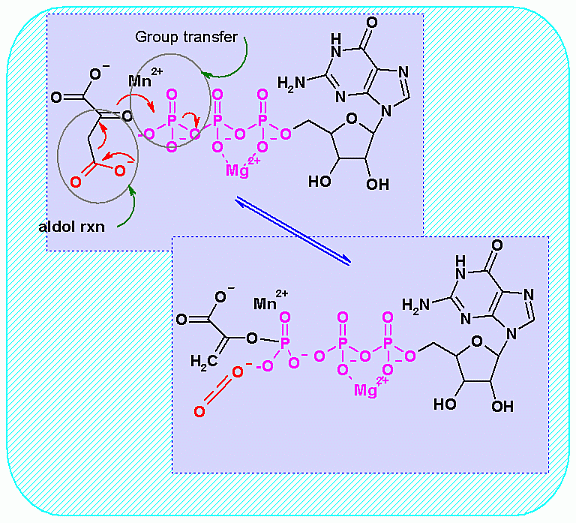
|
|
|
Picture of Enzyme with substrate |
|
|
|
|
|
|
|
Phosphofructokinase
PFK-1 CHIME representation |
|
*= These are concentrations obtained for one set of conditions. These will change as physiology and activity change.