 Fructose BisPhosphatase Information
Fructose BisPhosphatase Information Fructose BisPhosphatase Information
Fructose BisPhosphatase Information
|
Enzyme Name |
Fructose-1,6-Bisphosphatase |
|
|
Reaction Catalyzed |
Hydrolysis of phosphate from C1 Fructose-1,6-BisPhosphate
|
|
|
Reaction Type |
Hydrolysis |
|
|
Rationale |
In order for a metabolic pathway to run in the desired direction, the total ΔGo' of the pathway must be negative (favorable). For this reason glycolysis and gluconeogenesis cannot simply be the reverse of each other. Some reactions MUST be different. This is one of the three that falls into this category. The other reactions of glycolysis that must different are catalyzed by hexokinase, phosphfructokinase, and Pyruvate Kinase. Eack of these glycolysis reactions has a counterpart in the gluconeogeneisis pathway that is different. This is one of those reactions. It is the counterpart to phosphofructokinase (PFK-1). Where PFK-1 put a phosphate on Fructose in an ATP dependent fashion, This enzyme, Fructose-1,6-bisphophatase, uses hydrolysis to remove a phosphate from C1 of fructose-1,6-bisphosphate. this step in gluconeogenesis is to remove phosphate, in a hydrolysis reaction. Hypothetically speaking - Why it just a hydrolysis. why not a group transfer to ADP to make more ATP? Think thermodynamics here... what is the DGo' for this hydrolysis reaction? and then what is the DGo' for synthesis of ATP from ADP + Pi. If these Standard Free Energies are added where does that leave us? |
|
|
Pathway involvement |
Gluconeogenesis ONLY |
Fructose-1,6-BisPhosphatase is not part of the glycolysis pathway. In glycolysis a separate enzyme (Phosphofructokinase) adds a phosphate onto Fructose-6-Phosphate and is ATP dependent. The reaction catalyzed by Fructose-1,6-BisPhosphatase is a hydrolysis which in water is extremely difficult to reverse- because water is in very high concentration. |
|
Cofactors/Cosubstrates |
Mg2+ is required for this enzyme at least in the bacterial version isolated from E. coli |
|
|
DGo' |
-17 kJ/M |
Starting from standard state and allowing the reaction to come to equilibrium the product concentrations would end up ~600 times higher than the substrates. This is a good way to keep the gluconeogenesis pathway going and to ensure that glucose is produced when necessary. |
|
Keq |
|
|
|
Comments |
Let's consider why hydrolysis reactions are very difficult to reverse in a water environment. First the ΔGo' greatly favors product formation (Frucrose-6-Phosphate and phosphate in this case). But we also know that either very high product or very low substrate concentrations can make the observed Free Energy go the other way. What is always left of these calculations however is the concetration of water. Water concetration is pretty constant and very high - somewhere in the 45-50 M/l range. Water is in effect a substrate in a hydrolysis reaction. Its high concentration makes it difficult to reverse a hydrolysis reaction. These arguments do not necessarily apply in your Organic Chemistry course where reactions might be carried out in an organic solvent where water concentration is low. There is vert little stated about the mammalian enzyme in the text. All the mechanism material presented on this page is for a bacterial enzyme. There are two or four identical subunits in the active enzyme. This is an allosterically (literally means another site) regulated enzyme. Onr of the key regulation points between glycolysis and gluconeogenesis. MUCH more about this in moule 8. For now just bear in mind that the kinetic properties of this enzyme can be greatly altered by compounds binding to a site that is well removed from the active in the protein structure. This enzyme is fructose-1,6-bisphosphatase. There is another fructosebisphosphatase (F2,6BPase) that is not involved in the metabolic pathway. This a a separate enzyme that hydrolyzes a phosphate from C2 of Fructose-2,6-bisphosphate to produce Fructose-6-bisphosphate. Fructose-2,6-bisphosphate is not metabolized, but rather serves ONLY a regulatory function. We will learn much more about this enzyme and the role of fructose-2,6-bisphosphate in Module 8. |
|
|
Substrate Concentrations* |
||
|
S1= |
Fructose-1,6-bisPhosphate |
0.00001 mM |
|
S2= |
||
|
P1= |
Fructose-6-Phosphate |
0.014 mM |
|
P2= |
Pi |
1 mM |
|
DG for these conditions |
 |
|
|
-8.6 kJ/M |
||
|
Mechanism for Chemistry |
 |
|
|
Mechanism for Enzyme |
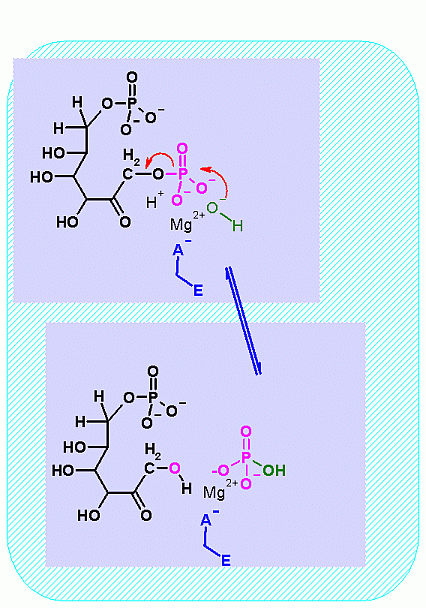
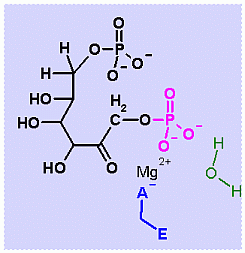 Fructose-1,6-BisPhosphatase. Animation of a Fructose-1,6-BisPhosphatase reaction Blue: represents the enzyme. THe EA- is an Aspartate from the enzyme active site in it's basic (deprotonated) state that ligands to the Mg2+. The phosphate is also liganded to the Mg2+. It is the Mg that aids in ionizinf the water molecule that attackes the phosphate group. "Start" begins an animation of the isomeriation reaction. It proceeds through the reaction in the "forward" direction and then "backwards" again. Note how the Mg ion is involved. "+" increases spped while "-" decreases the animation speed. You may also step through the reaction using "next" or "previous"
Fructose-1,6-BisPhosphatase. Animation of a Fructose-1,6-BisPhosphatase reaction Blue: represents the enzyme. THe EA- is an Aspartate from the enzyme active site in it's basic (deprotonated) state that ligands to the Mg2+. The phosphate is also liganded to the Mg2+. It is the Mg that aids in ionizinf the water molecule that attackes the phosphate group. "Start" begins an animation of the isomeriation reaction. It proceeds through the reaction in the "forward" direction and then "backwards" again. Note how the Mg ion is involved. "+" increases spped while "-" decreases the animation speed. You may also step through the reaction using "next" or "previous"
In describing the reaction in the pictures at the right, the Mg ion is coordinated to the enzyme ASP and to the phosphate on C1. water also coordinates and as a result its pK changes drastically an ionizes more easily. The resulting HO- can then attack the phosphate to begin the hydrolysis. Compare the animated reaction to the "arrow pushing" scheme at the right. See if you can correlate the electron movement in the animation to the arrows in the static picture above. |
|
|
Pictures of Enzyme |
|
|
NOTE: Apparently there is no structure of a mammalian fructose-1,6-bisphosphatase. These pictures and the CHIME version below are from a an enzyme isolated from E. coli.
|
||
|
Glucose-1-Phosphatase CHIME representaion | |
*= These are concentrations obtained for one set of conditions. These will change as physiology and activity change.