 Hexokinase Information
Hexokinase Information Hexokinase Information
Hexokinase Information
|
Enzyme Name |
Hexokinase |
|
|
Reaction Catalyzed |
ATP dependent phosphorylation of Glucose
|
|
|
Reaction Type |
Group Transfer |
|
|
Rationale |
Most cellular metabolites are charged. The reason is to maintain them inside the cell. uncharged molecules, like glucose for instance, are free to diffuse through the cell membrane, but charged molecules cannot diffuse through the membrane. Remember the "core" of the membrane is hydrophobic. Charged molecules and hydrophobic ones do not mix well. A good source of charge at pH 7 will be phosphate groups. Once a cell begins the metabolic processes on a specific molecule it would be a shame if it left the cell and went somewhere else. Like money in your bank account, you sure do not want it "leaking" to other accounts in the bank! So the first step in glycolysis is to put a phosphate on the glucose, in an ATP dependent group transfer. Why does it NEED to be an ATP dependent group transfer? why not just put a phosphate on using a condensation reaction (reverse of a hydrolysis)? Think thermodynamics here... What do we know about the thermodynamics of hydrolysis reactions How many places on glucose COULD the phosphate be transferred to? It is important to recall the mechanism of group transfer reactions here. COULD it be transferred to C6 (the alcohol at the "top"); to C1 (the aldehyde on the right)? Why or why not? |
|
|
Pathway involvement |
Glycolysis ONLY |
Hexokinase is not part of the gluconeogenesis pathway. In gluconeogenesis a separate enzyme (Glucose-6-Phosphatase) hydrolyzes a phosphate from Glucose-6P and is not ATP dependent. Rather it performs a simple hydrolysis reaction to cleave off phosphate. |
|
Cofactors/Cosubstrates |
ATP is a cosubstrate; ADP is a coproduct |
|
|
DGo' |
-16 kJ/M |
Starting from standard state and allowing the reaction to come to equilibrium the product concentrations would end up ~600 times higher than the substrates. This extremely favorable Standard Free Energy is helpful to get the entire pathway rolling in the right direction. |
|
Keq |
~600= |
|
|
Comments |
Why is ATP required? What would the DGo' for putting a phosphate on glucose be if we used inorganic phosphate (reversal of a hydrolysis reaction) instead of ATP? It would be +15 kJ/M. Which direction would be favored then? |
|
|
Substrate Concentrations* |
||
|
S1= |
Glucose |
5mM |
|
S2= |
ATP |
2mM |
|
P1= |
Glucose-6-phosphate |
0.83 mM |
|
P2= |
ADP |
0.14 mM |
|
DG for these conditions |
 |
|
|
-27 kJ/M |
||
|
Mechanism for Chemistry |
 |
|
|
Mechanism for Enzyme |
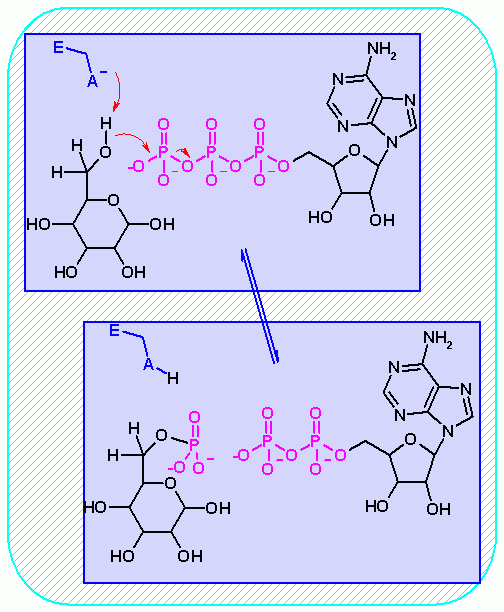
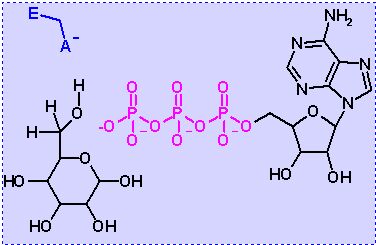 Hexokinase. Animation of an Hexokinase reaction Blue: represents the enzyme. THe EA- is the Aspartate from the enzyme active site in it's basic (deprotonated) state. "Start" begins an animation of the isomeriation reaction. It proceeds through the reaction in the "forward" direction and then "backwards" again. Note how the enzyme is involved. "+" increases spped while "-" decreases the animation speed. You may also step through the reaction using "next" or "previous"
Hexokinase. Animation of an Hexokinase reaction Blue: represents the enzyme. THe EA- is the Aspartate from the enzyme active site in it's basic (deprotonated) state. "Start" begins an animation of the isomeriation reaction. It proceeds through the reaction in the "forward" direction and then "backwards" again. Note how the enzyme is involved. "+" increases spped while "-" decreases the animation speed. You may also step through the reaction using "next" or "previous"
|
|
|
Pictures of Enzyme |
|
|
|
||
|
Hexokinase CHIME representaion | |
*= These are concentrations obtained for one set of conditions. These will change as physiology and activity change.