 |
 |
Chemical structure of tetrahydrofolate
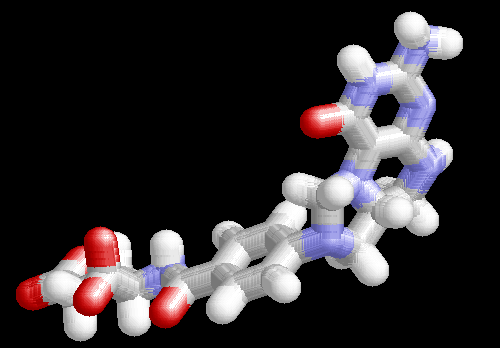
Between the two nitrogens circled a carbon atom can be added in varying redox states.
|
3D representation of the methylene derivative of THF ... A CH2 group is bound between the two "active site" nitrogens |
|
Vitamin B9 Dependence: Humans do not synthesize Folic Acid (The precursor of tetrahydrofolate) and therefore require its intake as part of the diet. Folic Acid is one of the (many) B types typically called vitamin B9. You may read much more about this and issues regarding its synthesis and deficiencies here.
|
Intake of additional folate at the beginning of pregnancy has been found to be associated with reduced risk of certain birth defects. The reasons remain unknown... it is just an association at this point.
|
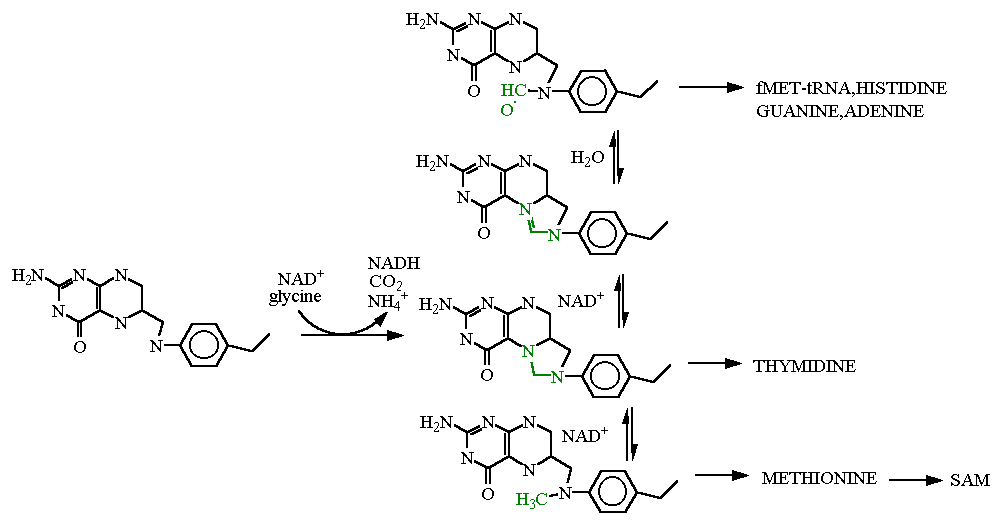
THF is a single carbon atom carrier molecule. It can cary the carbon in any of the oxidation states from methyl (-CH3), methylene (-CH2-), formyl (>C=O) or formic (-COOH). The single carbon is used for synthetic purposes. It is of particular use in the synthesis of the nucleotide bases |
 |
|
In the reaction schemes above tetrahydrofolate reacts with glycine to make the methylene (-CH2-) (shown in green) is added between the two "active site" nitrogens. This version is eventually used in the synthesis of Thymidine. Alternatively, it can be reduced using NADH (going down) to the Methyl derivative (-CH3). This is subsequently used to put the terminal methyl group on methionine which can then be added to adenosine to make S-adenosylmethionine as well. Going the other direction (up from methylene) it can be oxidixed using NAD+ to make the formyl (>C=O) derivative. The top two are the same oxidation state and differ only by addition of water (that is supposed to be a double bonded oxygen in green there). The formyl derivative is subsequently used in the synthesis of histidine, adenine or guanine.
|


