The Aldol reaction is the only way that we will discuss in this course for making and breaking carbon-Carbon (C-C) bonds. Making a C-C bond is called an Aldol Condensation while breaking a C-C bond is called an Aldol cleavage. These are NOT two separate reactions, but are in fact just reverse reactions. In this course, these are frequently combined into the term "Aldol Reaction"
The molecule MUST be appropriately arranged for a cleavage reaction. There are two requirements.
- it must contain a ketone/aldehyde
- AND the second carbon away must be either an alcohol
or an acid
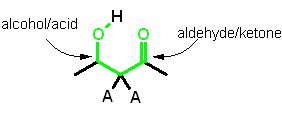 Molecule conditions for an "Aldol Cleavage"
Molecule conditions for an "Aldol Cleavage"
"A" = anything
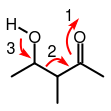 electron movement to initiate Aldol Cleavage
electron movement to initiate Aldol Cleavage
If these conditions are satisfied, the chemistry begins at the ketone. Something must pull one pair of electrons from the double bond up toward the oxygen atom. This MUST involve a good electron
 Where "EN" stands for the C=C and "OL" stands for the alcohol
Where "EN" stands for the C=C and "OL" stands for the alcohol
Transition State Intermediate. This "pulling" of electrons toward the oxygen atom generates an additional action - another pair of electrons must come from the neighboring carbon to fill the void. Effectively, the transition state now looks like a Carbon-Carbon double (EN) bond with an alcohol (OL) -single bond from Carbon to Oxyen and all other bonds are to Carbon/Hydrogen- on it called an
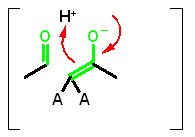 Electron movement away from the transition stateCompleting the reaction. As stated above the transition state is very unstable and short lived (the definition of transition state). The electrons cannot stay where they for long and "collapse" back toward a more stable position. They can completely reverse the original movement and get back to starting material (boring) or instead of going back to the carbon can pick of the H+ and produce product.
Electron movement away from the transition stateCompleting the reaction. As stated above the transition state is very unstable and short lived (the definition of transition state). The electrons cannot stay where they for long and "collapse" back toward a more stable position. They can completely reverse the original movement and get back to starting material (boring) or instead of going back to the carbon can pick of the H+ and produce product.
 two different molecules through cleaving a C-C bond
two different molecules through cleaving a C-C bondThe reverse reactionThe reaction sequence shown above is for the Aldol cleavage. The Aldol Condensation is exactly the reverse reaction proceeding through the same enol intermediate... ending of course with the longer molecule.
Electron pullers - catalysis
As shown above it is a pretty slow reaction. it must be catalyzed to demonstrate an appreciable rate. Enzymes always have some way to initiate the electron mocement. The initial electron motion that starts the reaction requires something fairly drastic. Some kind of "device" that will make the electrons move toward the transition state. usually a positive charge of some kind
case 1: The Schiff's Base (imine)
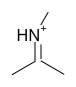 Imine: Enzyme makes covalent bond with substrate at the ketone - effectively replacing the "O" atom with "N". this places a "+" charge at the site. This is generally done with the LYS side chain.
Imine: Enzyme makes covalent bond with substrate at the ketone - effectively replacing the "O" atom with "N". this places a "+" charge at the site. This is generally done with the LYS side chain.
The "stabilization" of the enol transition state in this case requires a a strong electron "puller". Enzymes provide this stabilizer with a suitably electrophilic (something that 'attracts' electrons like a positive charge for instance) catalytic group such as Schiff's Base (imine covalent intermediate). This imine is made during the course of the reaction. The aldehyde/ketone of the molecule to be reacted combines with the amine of a lysine sidechain.
case 2: metal ion
Another possibility a divalent metal ion such as Mg2+, Mn2+ or Ca2+. In this case the enzyme contains one of these metal ions in the active site.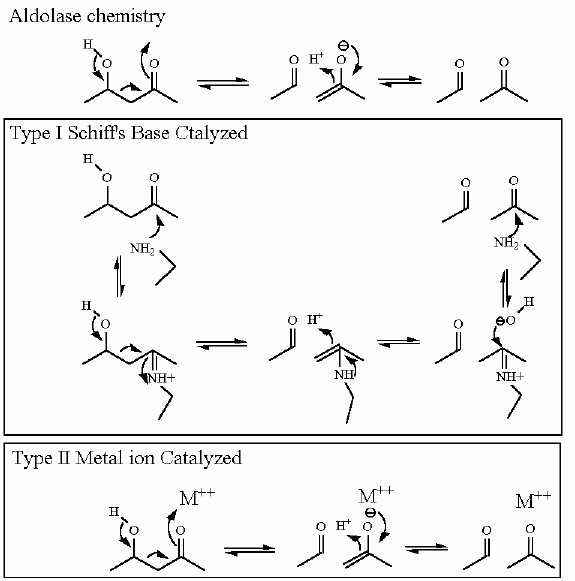
See the arrow pushing in motion for the aldol reactions
Thiamine Dependent Aldol Reaction
There is ONE exception to the "2 carbon rule" stated above. A carbonyl (ketone or aldehyde) and an alcohol (or acid) are still required, but they can be adjacent carbons  if and only if the vitamin B1 derived thiamine pyrophosphate is used as a cofactor. In the cases where the C-C bond between an adjacent ketone and alcohol/acid is broken the cofactor thiamine pyrophosphate is required ... As described on the linked page, thiamine pyrophosphate makes a covalent bond to the compound at the carbonyl- then this complex has an imine two carbons away from the alcohol/acid. This makes the reaction look very similar to the Schiff's Base catalyzed reaction described above.
if and only if the vitamin B1 derived thiamine pyrophosphate is used as a cofactor. In the cases where the C-C bond between an adjacent ketone and alcohol/acid is broken the cofactor thiamine pyrophosphate is required ... As described on the linked page, thiamine pyrophosphate makes a covalent bond to the compound at the carbonyl- then this complex has an imine two carbons away from the alcohol/acid. This makes the reaction look very similar to the Schiff's Base catalyzed reaction described above.
