|
Enzyme Name |
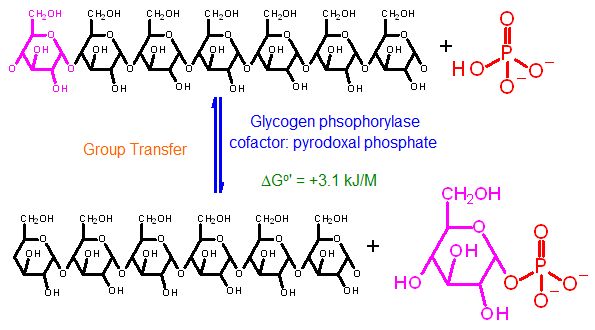
Glycogen Phosphorylase | |
Reaction Catalyzed |
phosphorolysis of glycogen (cleavage of one glucose from the C4 end using phosphate as the attacking molecule instead of water)
| |
Reaction Type |
group transfer Reaction | |
Pathway Involvement |
Glycogen Breakdown |
|
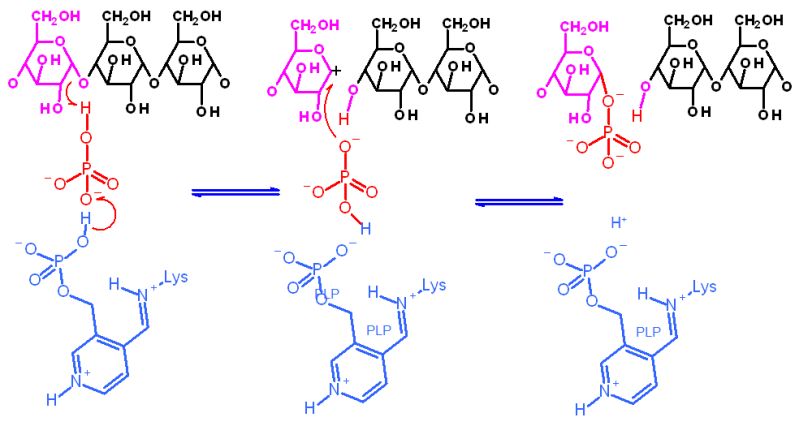
Cofactors/Cosubstrates |
Pyrodoxal phosphate - but used in an unusual manner (see mechanism below). | |
|
Rationale |
Complicated reasoning for this one... 1. There are two possible fates for glucose cleaved from glycogen. A.If used for the cell's metabolism, the advantage to using phosphate to cleave the glycogen polymer is obvious. The first step in glucose metabolism would be to use an ATP to put a phosphate on. - no need for that now as there is already a phosphate. Thus is saves an ATP for every glucose. (recall that it took 3 to put the glucose onto the polymer inthe first place.) B.A little toughter to rationalize if the glucose is to be exported. The phosphate will have to be removed before being released from the cell. Why not just hydrolyze the terminal glucose off the glycogen polymer. it would have better thermodynamics (for this one reaction ) and bypass the other steps. I guerss it is just best to have one enzyme perform the function regardless of intended fate. Afterall the cell cannot "know" ahead of time where the glucose will go 2. Thermodynamics also presents a potential problem. For this we need to look at the whole system. There are three reactions in the glycogen breakdown
First there is a much higher concentration of PO4 than of glucose-1-P (see thermodynamics section) therefore the ΔG is actually negative. Otherwise one must go to the third reaction in the sequence to get to thr driving reaction. Again the question remains - why not just hydrolyze the terminal glucose off the polymer and ship it out. It would have favorable thermodynamics AND not require so many steps. - We just have to suggest that one way was developed and that is the one that is used for all.... > | |
|
ΔG°' |
+3.1 kJ/M |
The Standard Free Energy favors glycogen formation from glucose-1-phosphate. |
Keq |
0.33 = [glucose-1-phosphate] / [PO4] - (glycogen concentration does not change and can therfore be ignored in this equation) | |
Comments |
Starting from standard state and allowing the reaction to come to equilibrium the reaction thermodynamics actually favors glycogen formation. The [PO4=] in cells is far higher than the concentration of glucose-1-phosphate. so the ΔG for the cell conditions is actually negative.
| |
|
Mechanism for Chemistry |
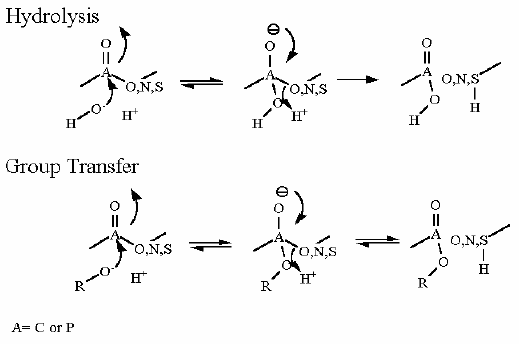 | |
Mechanism for Enzyme |
Hover your mouse over a step to reveal a description | |
|
Picture of Enzyme with substrate |
 |
|
|
Click an atom to diplay it's identity here | |
|
Messages about the currently highlighted features |