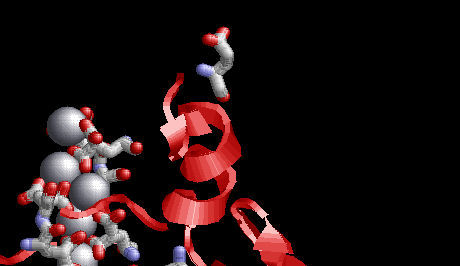 |
|
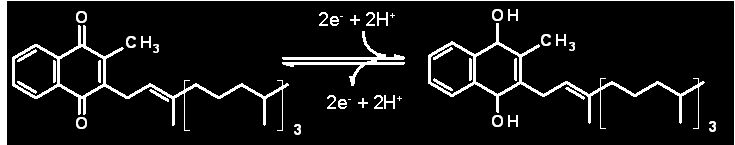
Chemical structures of phylloquinone (Vitamin K) in the Oxidized and Reduced forms
Two electrons and two protons are involved in the conversion |
Vitamin K Dependence: Humans do not synthesize phylloquinone and therefore require its intake as part of the diet. You may read much more about this and issues regarding its synthesis and deficiencies here.
|
Phylloquinone is a lipid soluble cofactor that is essential in the blood clotting cascade in that is makes crucial modifications of some of the enzymes. |
This modification is to alter some glutamate amino acids with ANOTHER organic acid on the γ Carbon. the result is a modified amino acid called γcarboxy-glutamate 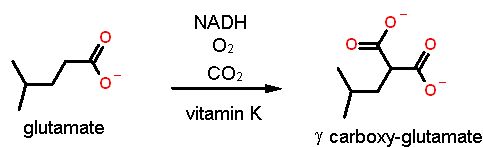 |
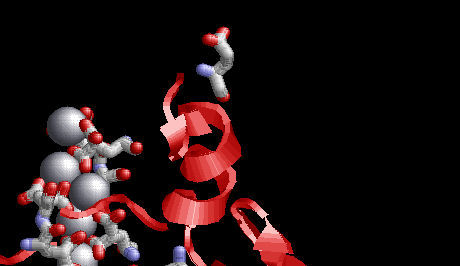
Protein with an unmodified glutamate
| 
Same protein with the modified glutamate - now γ-carboxyglutamate
|
| The point for this modification is that the modified glutamates now bind calcium very tightly. This has an unusual effect. Normally, at physiological pH glutamate is negatively charged as the organic acid is unprotonated. &gamma-carboxyglutamte would then carry 2 negative charges - one for each organic acid. But then as Ca2+ (calcium is always +2) the overall charge in the whole group (amino acid side chain + calcium) becomes zero. The negative charges of the glutamates prevent the protein from binding to the surface of a membrane. But with the calcium bound, it may now form this complex which is more active than the non-membrane bound for of the enzyme.
|
Many glutamates on the same protein molecule can be modified. thus generating many places where calcium binds. In the image below the calcium ions are purple spheres

same protein as above but showing several places where the glutamates have been modified - each binding calcium (purple spheres)
|
this reaction is cataltized by an enzyme and takes place in a sequential fashion
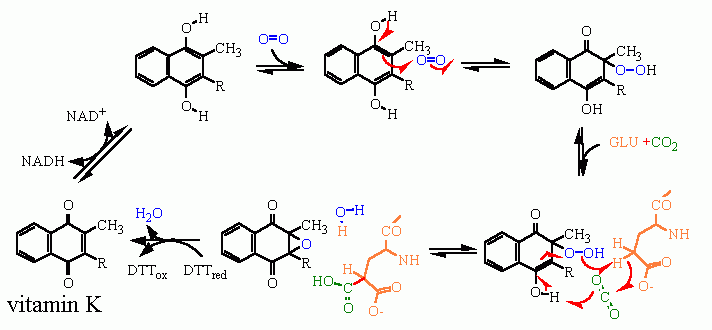
The reaction sequence begins in the lower left of this picture with the oxidized vitamin K. It is first reduced by NADH - then reacts with O2 to generate a "peroxide". This is a reactive species that can remove a H· (hydrogen radical) from the γ carbon of glutamate. Leaving behind a radical that reacts with CO2. Some clean up of the various radicals returns the original vitamin K to go through another cycle.
|