This is the dominant form
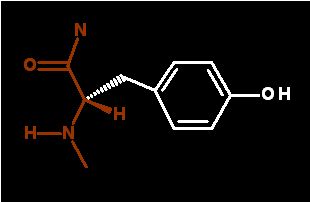
There is very little of this under normal conditions
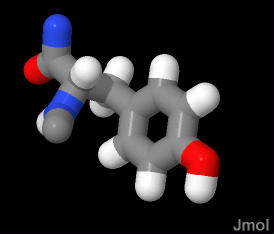

Large bulky aromatic amino acid side chain.
Due to the aromatic character AND the terminal hydroxyl group this side chain has a large light absorption property at 280nm.
Considerably more polar than pheylalanine. The hydroxyl group is an OH hydrogen bond donor and acceptor.
The -OH group under normal conditions has a pK of about 10 (note this is considerably lower than that for SER and THR - this is due to the aromatic character of the ring). In rare cases, the pK can be shifted by a large degree in some cases such that the ionized form can be seen in some proteins.
It can also be used in some cases as a metal ligand. If the metal is Fe or Mn then the protein will have a purple color because of the metal - tyrosine charge transfer.
In the demonstration below, the TYR is in a section of random coil and is partially buried in the protein with the hydroxyl group peeking out into water.
  Atom Label Description | |
|
Click an atom to diplay it's identity here | |
|
Messages about the currently highlighted features |