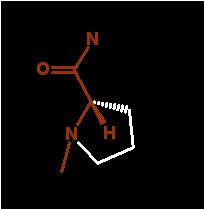
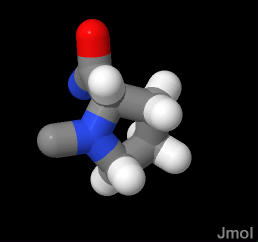

NOTE: all three bonds to the mainchain "N" are to Carbon. There is no N-H bond. Therefore this amino acid cannot form H-bonds to others in the chain.
This is the only amino acid in which the side chain is makes a ring with the main atoms. This constrains the phi/psi angle set immensly.
Proline is rarely seen in a helix and ONLY at the N-term end of one at that. The reason is NOT that proline cannot bend into the proper angles, in deed it can. But restricts the phi angle of the previous amino acid into angles not consistent with an α-helix and because the amine group of the main chain cannot form the required H-bond that is a necessary part of the α-helix;
In the demonstration below, the proline is in a random coil section of the peptide
  Atom Label Description | |
|
Click an atom to diplay it's identity here | |
|
Messages about the currently highlighted features |