|
|
| Name | 3 Letter | 1 Letter |
Cysteine |
CYS |
C |
|
Protonated (acid form - neutral)mostly dominant --- Unprotonated (base form - negative)
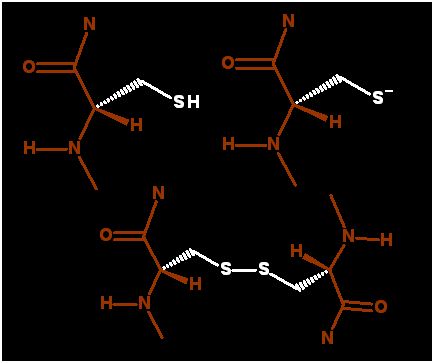
Two oxidized Cysteines
sulfurs form disulfide bridge |
 |

 |
|
Drawn as if part of protein to emphasize the sidechain properties
| |
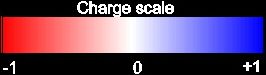
The "Sidechain Polarity" button draws an an envelope around the sidechain that is colored by charge according to the scale above. |
|
| pKR in H2O= 8.2 |
Free Amino Acid Mass = 121 g/M |
|
| Probability of being found in a: |
| α-Helix |
β-Sheet |
β-Turn |
| 55% |
75% |
70% |
| Special Attributes |
|
Polar amino acid can act as a hydrogen bond donor or acceptor, but generally the hydrogen bonds made with sulfur are considered to be weaker than those made with oxygen.
If the sulfur atom of one cys lies next to another cysteine residue then there is potential for both sulfurs to become oxidized and form a disulfide bridge.
This has the effect of providing covalent crosslink bond across regions of a protein structure.
Cysteines can be found on either the surface of a protein or buried inside. In the demonstration below, the cysteine is at the end of an α-helix and is buried
|
|
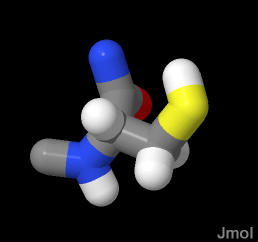
In the demonstration below you may "left click and drag" to rotate the molecule "SHIFT left click and drag (up or down)" to make smaller or bigger
Click on the white squares in succession to turn or/off identifying features. Some text describing the issues is in the lower right text box |
|
|


Atom Label Description

|
|
Click an atom to diplay it's identity here
|
|
Messages about the currently highlighted features
|
|
| Jmol: an open-source Java viewer for chemical structures in 3D. http://www.jmol.org/ |
|





