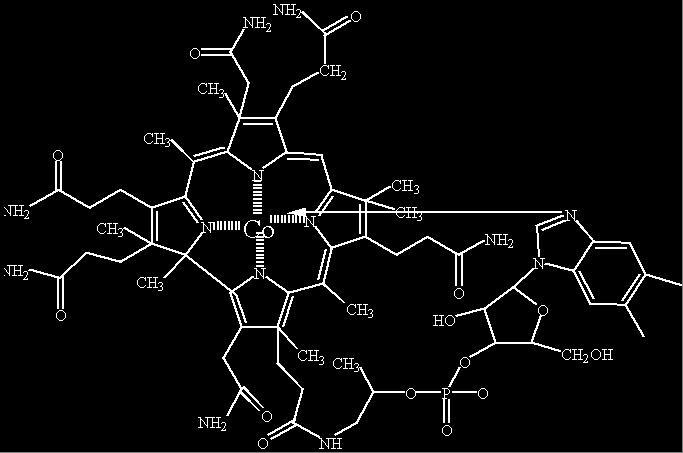
The long arrow between N and Co is a bond. It is drawn this way in an attempt to display it in two dimensions
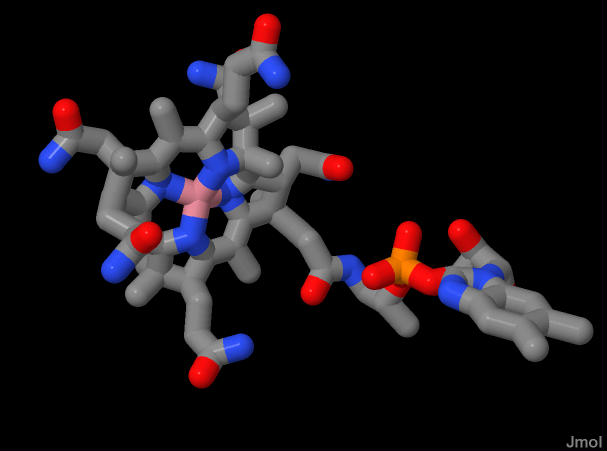
In this particular view the adenosine ring has dissociated and is not bonded to the cobalt.

 |
 |
| Chemical Structure of Cobalamine The long arrow between N and Co is a bond. It is drawn this way in an attempt to display it in two dimensions |
3D representation of cobalamin. In this particular view the adenosine ring has dissociated and is not bonded to the cobalt. |
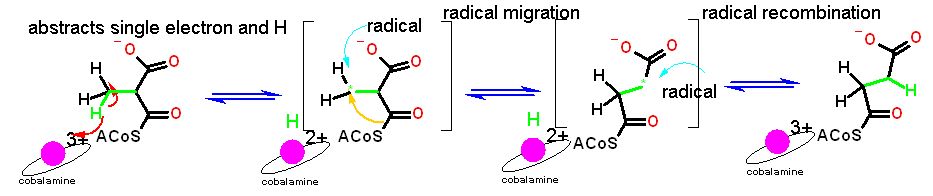
| Cobalamine is a cofactor that is invovled in unusual reactions. Its siganture reaction is the migration of a methyl group from one unactivated carbon to an adjacent unactivated carbon. This is radical (1 electron) chemistry that is beyond the scope of this course | |
 | |
| In the reaction above, methylmalonyl-CoA (on the left) is converted to succinyl-CoA (on the right). This reaction involves some unique chemistry that is enable by cobalamine. The cobalt extracts a single electron and H from the methyl group (there is actually an intermediate step not shown) - generating a radical - a lone unpaired electron - on the carbon atom. Strange things tend to happen with radicals. In this case the radical migrates from teh methyl carbon to the methylene carbon next door. As this happens, the carbons attached must move as well. The thioester carbon migrates to where the radical was. The radical is now on a methylene carbon but is still unstable. It takes the electron and H back from cobalamine and completes the conversion to succinyl-CoA. | |