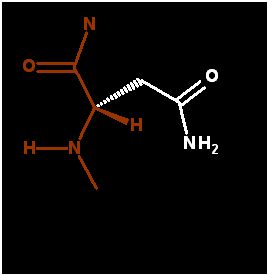
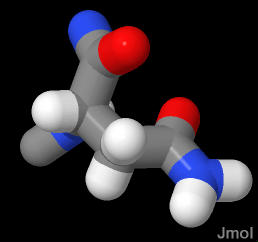

Asparagine ends in an amide group that is very similar to the peptide bond. It too is very polar and planar. Very good a forming H-bonds. Since it ends in an amide (not an amine) this group is NOT ionizable (does NOT pick up or lose a proton)
As the terminal amide in aparagine is polar (just like the peptide bond - which is also an amide bond), it is frequently exposed to water as it is in the demonstration below. In this case, the asparagine is found in a β-turn.
  Atom Label Description | |
|
Click an atom to diplay it's identity here | |
|
Messages about the currently highlighted features |