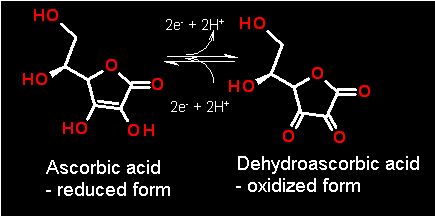
Two electrons and two protons are involved in the conversion
Ascorbic Acid is a water soluble anti-oxidant that is known to react with radicals in the body as they occur. The radical can form frequently as "mistakes" from oxygen metabolism in what are called "reactive oxygen species" (ROS). Oxygen is stable only as O2 and its four electron reduced version - WATER. All of the intermediate reduction states, O3- (superoxide), H2O2 (hydrogen peroxide) and OH· (hydroxide radical) are all extremely destructively reactive. The first thing they contact they will modify - sometimes in unpredictable ways. This could be proteins, DNA, lipids.. whatever. If it happens to be an Ascorbic acid molecule that is contacted first... excellent!
We will not see any direct examples of Ascorbic Acid utilization in enzyme reactions in this course. Check out the Wikipedia page above for some examples.
