

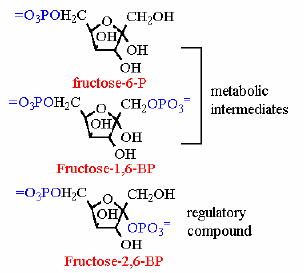
One of the important allosteric effectors (molecules that bind to an allosteric site to affect kinetic properties) in liver cells is fructose-2,6-bisphosphate (F26BP). This not a metabolic compound, but exists primarily for the purpose of connecting glycolysis and gluconeogensis to hormonal regulation (more detail coming about this in the next couple of pages). The enzyme that catalyes the synthesis if F26BP is separate from the phosphofructokinase-1 that is part of the metabolic pathway. Its sole funcion is is to produce this one compound - and degrade it again. This enzyme is unique in some ways. Recall that if there is an enzyme to put the phosphate on (using ATP of couse) then there must be another enzyme to hydrolyze the phosphate off of F26BP again. Indeed there is. It just so happens this time that BOTH of these enzyme activities reside on a single polypeptide - at two differnt active sites. (kind of two proteins spliced together)
This of course leads to some nomeclature problems.
| Reaction | Activity Name | Abbrev. | |||
| Reaction 1 | Fructose-6-P + ATP | 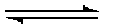 | Fructose-2,6-Bisphophate + ADP | phosphofructokinase-2 (places PO4 on C2 of fructose) | PFK-2 |
| Reaction 2 | Fructose-2,6-bisphosphate | 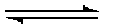 | Fructose-6-P + PO4= | Fructose-2,6-bisphosphatase | F26BPase |
| What to call the entire peptide then? | theroretically catalyzes both reactions (we will modify this in a minute) | phosphofructokinase-2 | PFK-2 / F26BPase or just one of these for convenience depending on activity | ||
Both activities on a single peptide that catalyze a potenital futile cycle, sounds like a bad idea
In general ONLY one of these activities is "active" at a time. This is a good thing since this single peptide could catalyze that "futile cycle" all by itself. The activities are modified by covalent modification with a phosphate group. More on how this gets there in the next two pages. Suffice it to say that It is placed on by an enzyme called "cyclic AMP dependent protein kinase" and removed by another enzyme (differnt polypeptide this time) called protein phosphatase. In the picture below, The yellow spheres represent the single polypeptide with the two activities PFK-2 and F26BPase; the * indicates the covalently modified peptide.
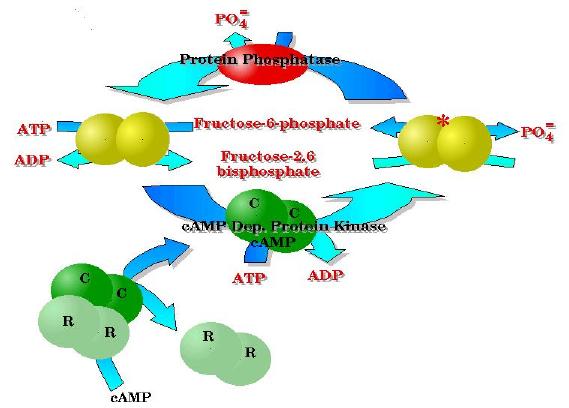 PFK-2 contains two identical subunits - each of course have both active sites (PFK-2 and FBPase-2) - represented by the yellow spheres.
PFK-2 contains two identical subunits - each of course have both active sites (PFK-2 and FBPase-2) - represented by the yellow spheres.