
Michaelis/Menten Enzyme Kinetics Returns
Most enzymes obey the standard Michaelis Menten kinetics scheme that we have already discussed in module 4. The
implications of this in the context of a metabolic pathway must now be discussed. Most of these enzymes have evolved for
the many millenia to have a Km near the substrate concentration that is "normally observed" in an individual
at rest. Enzymes are not generally running at a rate near their maximum but considerably less. As substrate
concentrations changes with changes in physiological conditions then the rate of flux through these enzymes can change
appropriately.
Let's consider two scenarios.
- An individual at rest
- An active individual but still with adequate glucose supply.
The first individual does not have much energy demand for muscular motion, therefore the use of ATP is not rapid. and
the need to make more ATP is not high. The need for oxidation of more glucose to make ATP is not high. There is
however a constant need for ATP synthesis to maintain the building of polymers, thermostasis and other regular
maintenance. While the demand for ATP is not high there is indeed some demand. The pathways should operate well but
there is no urgency.
The second indiviual on the other hand, has immediate need for fast synthesis of ATP since muscular motion is depleting
the supply as fast as it can be made. Since the demand is high we need the pathways to operate at higher rates overll to
increase the FLUX of carbon through the system. As the flux through the system increases the substrate concentrations of
each enzyme will increase.
If the substrate concentration was near or below the Km for the enzyme while at rest, then as the demand increases the
rate of substrate conversion the te "unregulated" enzymes will increase as well.
Shown is this picture is a graph representing the standard Michaelis Menten kinetics. The gray area shows a range of
substrate concentrations that might be observed depending on the physiological state.
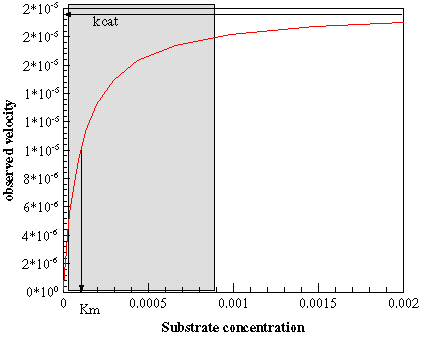 The vertical arrow indicates what the concentration of substrate might be
while at rest. As substrate concetration changes the rate of conversion from S to P aslo changes accordingly.
The vertical arrow indicates what the concentration of substrate might be
while at rest. As substrate concetration changes the rate of conversion from S to P aslo changes accordingly.
If the concetration of [S] averaged at "0.0015" Molar or so, this would not be the case. The enzyme is already
operating near its Vmax additional substrate would not appreciably increase the rate of conversion.
Since the average substrate concentration is ususally near Km we can obtain a rate of conversion nearly
proportional to and changes in substrate concentration without any input or alteration of any kind.
 The vertical arrow indicates what the concentration of substrate might be
while at rest. As substrate concetration changes the rate of conversion from S to P aslo changes accordingly.
The vertical arrow indicates what the concentration of substrate might be
while at rest. As substrate concetration changes the rate of conversion from S to P aslo changes accordingly.