

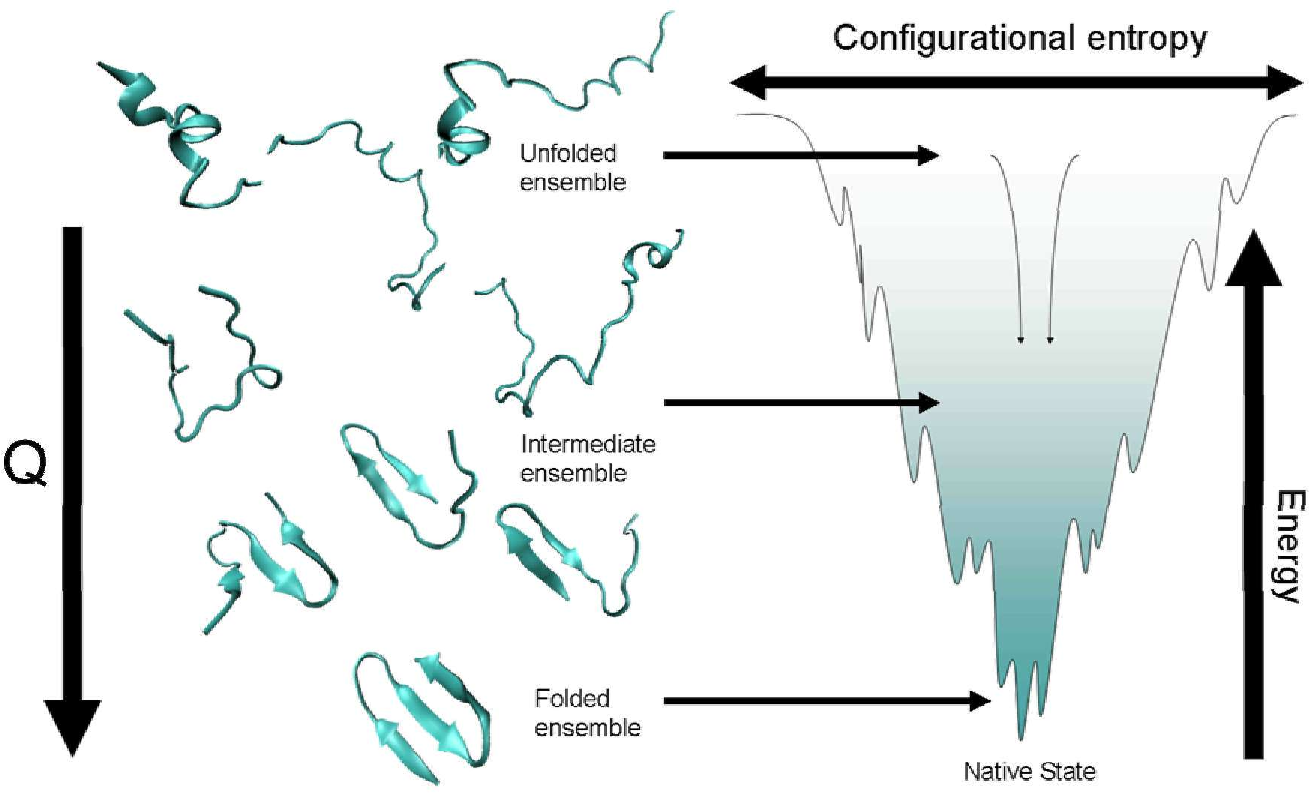
Way back in module 2, when we were discussing protein folding, I presented the picture to the right. What it represents is that unfolded proteins have a broard array of rapidly changing structures that are constantly sampling "new folding space". The interactions between groups od a protein are constantly alternating. some have a higher energy than others. But nature is lazy and the total energy associated with this state is quite high. As favorable interactions take place the energy of the system gets lower. This means that energy must be put back in to "undo" that favorable interaction. AS more favorable ineractions take place the energy falls even lower yet until one reaches the lowest point. At this lowest point in the "funnel" the protein is folded into its "final" structure.
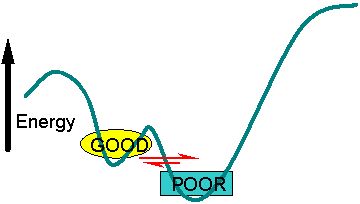 Energy landscape without Allosteric binding
The teal line represents the "bottom" of the energy landscape funnel shown at the top of the page. Two closely related structures are separated by a small energy barrier, but one has slighly lower energy and is therefore favored in the equilibrium. In this case it is a structure that has POOR catalytic properties.
Energy landscape without Allosteric binding
The teal line represents the "bottom" of the energy landscape funnel shown at the top of the page. Two closely related structures are separated by a small energy barrier, but one has slighly lower energy and is therefore favored in the equilibrium. In this case it is a structure that has POOR catalytic properties.
What is also apparent here is that while there may be a "lowest" point in the energy landscape, it is not much lower than another point. Add into this that no molecule is static but is constantly vibrating with bonds that are stretching and flexing.This means that atoms are constantly bumping into each other and giving their neighbors a little "kick".
This is the orgin of some proteins having a couple closely related structural forms. These two forms are very close in erergy, with one is slightly lower. The energy barrier between then is not very high so they interconvert rapidly. This is shown at the left.
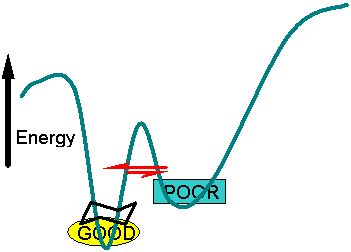 Energy landscape WITH Allosteric binding
The teal line represents the "bottom" of the energy landscape funnel shown at the top of the page. Two closely related structures are separated by a small energy barrier, but one has slighly lower energy and is therefore favored in the equilibrium. In this case a compound binding to the allosetric site or at the active site stabilizes the GOOD form lowering its energy. Now the equilibrium favors the GOOD form of the structure.
Energy landscape WITH Allosteric binding
The teal line represents the "bottom" of the energy landscape funnel shown at the top of the page. Two closely related structures are separated by a small energy barrier, but one has slighly lower energy and is therefore favored in the equilibrium. In this case a compound binding to the allosetric site or at the active site stabilizes the GOOD form lowering its energy. Now the equilibrium favors the GOOD form of the structure.
For some of these proteins a small compound binding to the active site or the alosteric site stabilizes one form ... lowering its energy relative to the other and also altering the equilibrium point between the two structures. In this case a small molecule binding to the allosteric site lowers the energy of the GOOD catalytic form.
The exact same priciples apply ro those enzymes that are covalently modified. The modification alters which structure has the lower energy - thus altering the equilibiurm between the structures
For the blood coagulation factors the issue is a little differnt. In this case there is one structure that can be observed, until the amide bond is hydrolyzed. Then the protein relaxes to a new (highly realted) structure that ha a lower energy. This new structure is allowed because of a change in restrictions on what conformations the structure can have.
Building things (blood clots for instance) always requires energy input. The energy released in the hydrolysis of the amide bond in fibrin allows it to rearrange to a lower energy that is amenable to protein molecules associating with each other. The release of energy from the hydrolysis of the amide bond is energy input into building the clot.
The energy was input into the system using a boatload of ATPs synthesizing all those amide bonds while the protein was being assembled at the ribosome.