


It is VERY important to recall that each of the steps during the amplification stage of the cascade, is catalytic. in each picture one "molecule" is shown converting one other molecule. But since each is catalytic it can actually convert MANY of the those molecules. End result.... Each enzyme can concert 1000's of the next enzyme in the cascade. By the time we get to the "end", many thousands of Thrombins can convert 1000s of thousands of fibrinogen to fibrin. The cascade generate an amplification of the signal so that only a few of the initiation contacts are required to generate a protein fibrin protein nework large enough to ensure slot formation and stopping of further blood loss.
Now that we have an initiation (thromboplastin from cells activating factor VII) and an amplification process, we have another critical problem to fix. The site of injury is fixed: the coagulations factors are water soluble AND moving as the blood plasma flows. It doesn't do much good to activate the system and then have the blood clot show up somewhere downstream. That would actually generate more problems than it solves. This particular problem can be solved by making the water soluble proteins complex with the platelets which are clumping and getting anchored to the site of injury by the collagen.
Once factor IX start to convert to factor IXa (the active form) It joins a complex of proteins and phospholipids that contains:
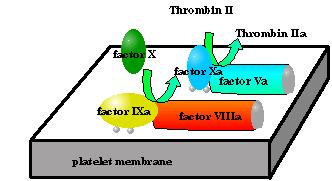 The "water soluble" factors are drawn as ovals;
The "water soluble" factors are drawn as ovals;The amide hydrolysis of factor X occurs most efficiently in this complex. In blood clotting the phospholipids are an actual membranes... mostly belonging to platelets. This enzyme, helper protein, phospholipids complex is instrumental in three activities:
What's the the function of each of these activities?
It has been well established that factor IXa is the "active catalyst" here. What are all the other "cofactors" (phospholipids, Ca2+ and factor VIIIa) for?
So the platelet aggregate provides an anchor point. There's still two problems to solve then...
Factor VIIIa and the Ca2+ provide the solution to to the anchoring. Factor VIIIa is a membrane bound protein that in and of itself does not have any catalytic activity, but does have a fairly high affinity for factor IXa. So using the same physical forces that maintain any quaternary structure Factor IXa associates with factor VIIIa.
The Ca2+ coordinates to the γ-carboxy glutamate side chains at we showed in module3. The Ca2+ can serve as a bridge between the proteins γ-carboxy-glutamates and the negative charges on the surface of the phospholipid layer of the membrane. So these too help to anchor Factor IXa to the membrane surface.
Proteins are inanimate objects... They do not actively seek out their binding sites, but associate with them once they have "blindly" bumped into them by random diffusion. Some of the "activated factors" may not associate with the membrane but continue downstream happily water soluble. This causes the obvious problem... If they are fully active they will start keep the clotting process going IN THE WRONG PLACE!
The factors IX and X though are not very active unless they are anchored to their appropriate factors which are anchored to the membrane. So indeed the phospholipids are "strictly" necessary to ensure the clotting process remain local!.