

 |
Select a picture above. These will initially be shown to 'morph' between the POOr and GOOD state. You may select to freeze one state or the other with the buttons below. (These button do nothing when the graph is selected above) |
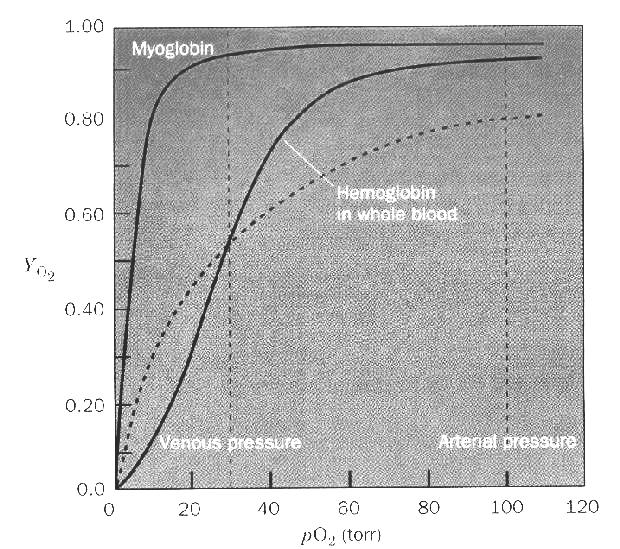
Hemoglobin is the prototypical cooperative protein. It contains 4 subunits: 2 α and 2 β. The α and β subunits bear similar sequences and VERY similar structures. This allows us to speak about hemoglobin as if it contains 4 identical subunits in terms of Cooperativity. Myoglobin, even though it carries the same function (bind oxygen) and bears a very similar structure to a hemoglobin subunit does not display Cooperativity because it is a monomeric protein. | |
Three different structures of hemoglobin are superimposed below.
The structures of the POOR and GOOD have an overall similar shape and fold, but differ slightly and in their oxygen binding affinity. In order to highlight the similarities and differences the structures are superimposed so that the heme molecules of the α and β subunits overlap as well as possible. What To Look For Between the POOR and GOOD forms there is a small, but significant, change in the heme conformation. In the POOR form the iron is slightly out of the plane of the heme, while in the GOOD form the iron is centered in the plane of the heme. While this appears small it is sufficient to alter the oxygen binding affinity. What is important is to trace how this change in one subunit is communicated to the neighboring subunits. As the iron atom changes position it alters the angle of the ring of the histidine attached to it. This results in a small motion of helix of which this HIS is part. amino acids at the end of this helix are essential in making contacts with a neighboring subunit. The main changes are observed between the α and δ subunits as well as the β and γ subunits. The interfaces between the α and β subunits as well as between the γ and δ are fairly rigid and do not change much. Hemoglobin consists of two different types of subunits that are similar in sequence and structure but not identical. An α/β pair has one of each as does a γ/δ pair. The interface between subunits of a pair is far more extensive than the interface between the pairs. For this reason Hemoglobin is frequently described as a dimer of dimers. Look to see how little the structure changes between the α/β and how extensive the change it between the α/δ subunits. What the Buttons Do The checkboxes are "features" that you may turn On or Off individually or all at once. The Radio buttons choose which structure(s) to display or compare. The bottom three checkboxes alter the color of a specific structure to make it easy to identify. | ||
|
Click an atom to diplay it's identity here | ||
|
Messages about the currently highlighted features | ||