|
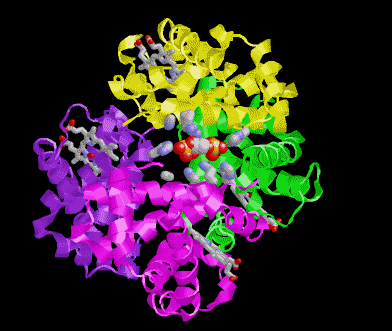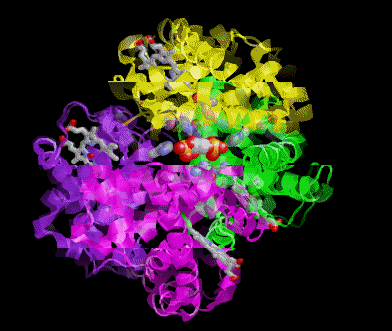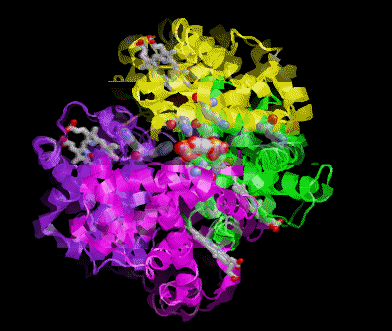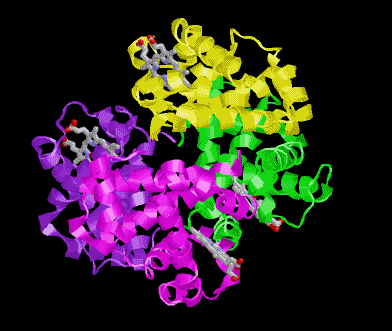
POOR (T) state = Oxygen is NOT bound to the heme
Picture morphs to GOOD (R) state with oxygen bound and then snaps to T state again.
In these pictures the the substrate 2,3 Bisphosphoglycerate (2,3BPG Shown as colored balls) is bound at the interface
between two subunits in the POOR (T) state. The binding is stabilized be several ionic interactions with the protein. As
the ribbon representation of the backbone of the protein morphs to the GOOD state, notice that the interface between the
subunits get smaller. A conflict arises between the amino acids and where the 2,3 BPG would be when the protein is in
the R state. Obviously the 2,3 BPG cannot be present in this site while the protein is in the R state.
Result 2,3BPG stabilizes the POOR oxygen binding form of hemoglobin in humans. This help to get oxygen released in
the tissues where oxygen concetration is low. If not for 2,3 BPG in the blood hemoglobin would remain mostly oxygen
bound in the veinous blood.
|