

It was previously indicated that in complexes I, III, IV electron transfer and H+ translocation are inextricably linked. In Complex V, ATP synthesis/ hydrolysis is inextricably linked to rotation of the γ subunit and H+ translocation.
The H+ gradient, however, can be fouled up with some drugs or experimentally by removing the system from the membrane.
A couple drugs that are known to uncouple electron transfer from ATP synthesis are:
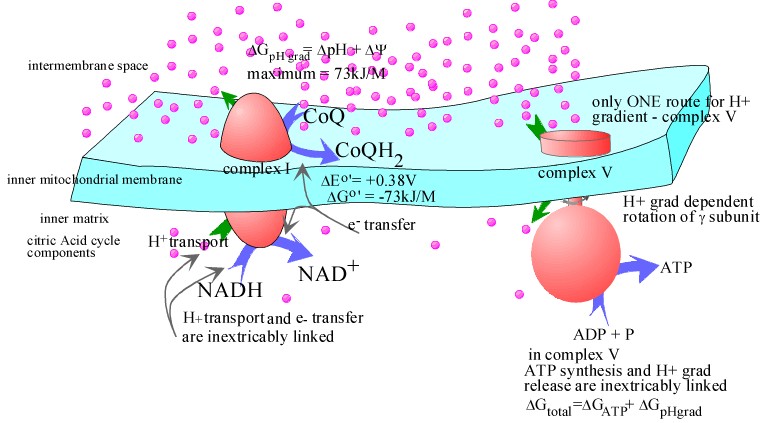
In the pictures above are represented two cases. The first case is the normal situation. (I an showing only complex I to aoid making the picture too cluttered). Here NADH transfers electrons to CoQ while pumping H+ from the inner matrix to the intermembrane space generating a H+ gradient. The H+ can only "attempt" to equalize by passing through Complex V and drivinr ATP synthesis. The purple dots are meant to represent the H+. In the first picture there are more on "top" than on the bottom.
If you click on the "Decoupler valinomycin present", the picture changes. The drug puts little holes in the membrane which allows the H+ gradient to dissipate via a route tht is indepdent of complex V. Noe the number of purple dots above and below the membrane are the same. and ATP is not synthesized. Note however, that complex I is still functional.. NADH still reduces CoQ.
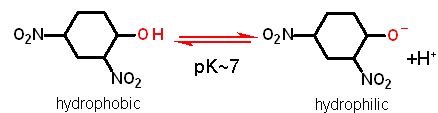
Dinitrophenol is a chemical with a pK~7. When it is protonated (at pH below 7 like that in the intermembrane space) it is hydrophobic and can cross membranes (even the inner mitochondrial membrane). When pH is above 7 (like in the inner matrix) it is negatively charged and can no longer cross the inner membrane. End result is that it can transport H+ across the inner mitochondrial membrane INDEPENDENT of complex V.... This decreases the ΔGproton gradient but does no work!
Valinomycin is a "donut shaped" short peptide that embeds itself in a membrane. In doing so it generates a hole in the membrane. The hole presents little barrier to ions (K+ in this case) going through it. When this happens to the inner mitochindrial membrane, positively charged potassium ions flood back into the inner matrix and the charge difference across the membrane is ruined. Thus the ΔGproton gradient decreases subtantially and it becomes too low to support ATP synthesis.
Regardless, these drugs provide an alternative route for the H+ "flow" back into the inner matrix and drastically slow ATP synthesis, BUT DO NOT HAVE MUCH EFFECT ON ELECTRON TRANSFER PROCESS.
Similarly, if the mitochondrial membranes are dissolved with a detergent (a common way to start purifying membrane proteins so that they can be studied) then the gradient cannot form at all. There is nothing to prevent free diffusion of H+ and nothing to force then to proceed through complex V.
In the 1960's (or there abouts) someone got the bright idea that dinitrophenol would be a great weight loss drug. It turns out to be extremely effective. Why would dinotrophenol be effective in weight loss? It is illegal to use it, however. It turns out that safety is a huge issue. While it is effective, some people think that a little is good ... more should be better. These latter folks developed dangerously high fevers. Why should this happen? Think Bear in mid that if the chemical energy is not trapped (using ATP synthsis) then it is lost as ????.
This problem is of course true for any compound that uncouples the electron transfer from ATP synthesis.