As alluded to two pages ago, a major piece of the puzzle is still missing. While glucose is completely oxidized and the resultant electrons have been satisfactorily disposed, There is still no connection to how to synthesize ATP from all the energy of sending electrons down the transport system.
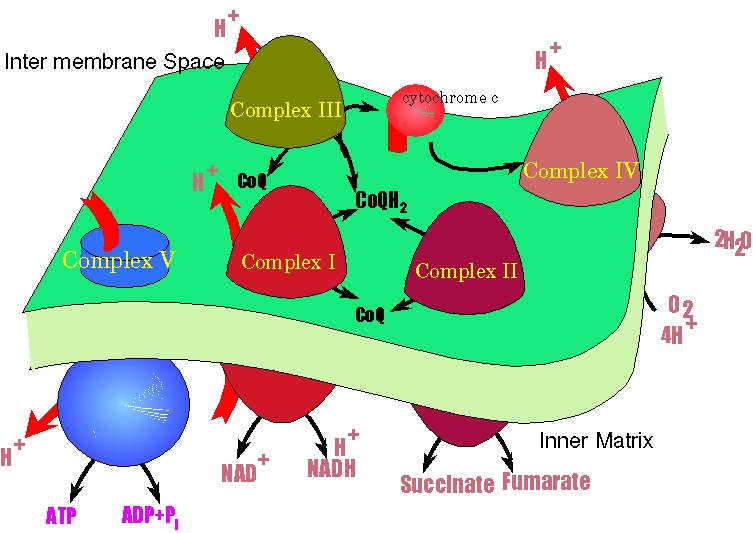
In three of the complexes (complex I, complex III and complex IV), the energy of the electron transfer is used to perform work; that of moving H+ from the inner matrix across the membrane into the inter membrane space. This reduces the H+ and the amount of positive charge in the inner matrix while simultaneously increasing the H+ and amount of positive charge in the inter membrane space. Consequently a H+ and an electrical (there is more positive charge outside the inner matrix than in it) gradient is developed.
The moving and H+ through complex I, complex III and complex IV are inextricably linked. One does not happen without the other. This means that the thermodynamics of electron transfer and the thermodynamics of the H+ gradient generation are linked as well. I will have more to say on this in a couple pages.
As with all thermodyanamic processes, it is reversible. A very high H+ gradient can make electron transfer go "in reverse".
As with any situation when a region of high concentration develops, diffusion should be the great equlaizer. It seems like the high concetration of H+ in the intermembrane space should just diffuse back to the low concentration place in the inner matrix. There is a problem with this, however,,, the membrane. Membranes have a hydrophobic core and generally prevent passage of charged substances. This includes even small things like H+. So the gradient cannot go away just be simple diffusion.
Rather. the H+ gradient can in turn be used to perform work also. In this case drive the synthesis of ATP from ADP and PO4=. This heppen because of yet another large protein complex (imagintively enough) called complex V.
The picture below is a representation of the process. The black arrows represent electron transfer, the red arrows represent H+ motion through the membrane at complexes I, III, IV and V. CoQ is the oxidized form of coenzyme Q while CoQH2 is the reduced form. Both complexes I and II take CoQ and make CoQ2. Complex III takes the CoQ2 and oxidizes it back to CoQ and sends thos electrons to cytochrome c. this latter then transfers electrons to complex IV to reduce oxygen to water. Of special note, the only place that allows H+ back into the inner matricx is complex V.