
In the previous module we found that we could net 2 ATP per glucose through a strictly, anaerobic fermentative (nonoxidative) breakdown of glucose to lactate (or ethanol + CO2). But there is much more energy to be obtained if glucose can be oxidized to carbon dioxide (CO2). In order for the complete oxidation of all the carbon to occur we need to reduce something---- for us that is molecular oxygen (O2).
This occurs in two separate steps
These are reactions in which there is a net change in the number of electrons in a molecule. Whenever electrons are taken away from one molecule (oxidation) then they MUST be added to a different one (reduction).
Whereas the non-oxidative pathways (glycolysis) takes place in the cell cytosol, the complete oxidation to CO2 as well as the reduction of O2 to water occur in the mitochondria of cells. Pyruvate from glycolysis is transported into the inner matrix of the mitochondria where these steps take place.
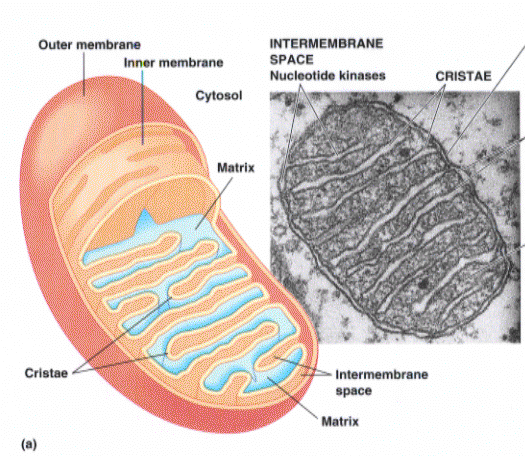 shown is a schematic representation of a cross section of a mitochondria. Also shown on the right side of this graphic is an electron micrograph of a cross section of a mitochondria organelle. There are two membranes around a mitochondria. 1. The outer membranes 2. The inner membrane. They differ markedly in their properties. The outer membrane allows passive diffusion of a wide variety of compounds due to the presence of a number of proteins that provide a pore through which to pass. The inner membrane on the other hand is highly selective. The ONLY molecules allowed to pass though this one are those for which there is a specific transport protein. The space in between the membranes is termed the Inter-membrane space. The space inside the inner membrane is termed the inner matrix.
shown is a schematic representation of a cross section of a mitochondria. Also shown on the right side of this graphic is an electron micrograph of a cross section of a mitochondria organelle. There are two membranes around a mitochondria. 1. The outer membranes 2. The inner membrane. They differ markedly in their properties. The outer membrane allows passive diffusion of a wide variety of compounds due to the presence of a number of proteins that provide a pore through which to pass. The inner membrane on the other hand is highly selective. The ONLY molecules allowed to pass though this one are those for which there is a specific transport protein. The space in between the membranes is termed the Inter-membrane space. The space inside the inner membrane is termed the inner matrix.
Notice, nothing in the above statements indicates anything about synthesizing ATP. This is of course the ultimate goal. Why bother if you do not get energy out of the system somehow. There is also the basic conundrum of how transfer of electrons from pyruvate to O2 (a redox process) can affect ATP synthesis from ADP + PO4= (a condensation (or reverse hydrolysis)). These reaction types are unrelated. I will have more to say about this in a few pages as this connection is essential.