
So let's see how these non-covalent interactions combine to make proteins fold. This is a complex process that is not completely understood (even by the current masterminds in the business). But I think it is instructive to take a look at how complex it COULD be if it were a completely random process.
The protein will start the folding process by rotation about several of the bonds that can do so... much like rotation of your arm about your shoulder, elbow and wrist. Just independently rotating these three joints can produce a wide variety of conformations in your arm. If you couls "click" your arm into 10 different independent positions for each your wrist, elbow and shoulder, there would be 103 possible combinations (that's 1000 possible conformations just for your arm). To put this into perspective, if you could move your arm into a new position (without repeating any) 10 times every second, then you could "visit" every possible conformation in 100 seconds (1 minute 40 seconds). It is therefore feasible for you to test every combination to find the most "comfortable".
Lets look at a small protein of about 100 amino acids and assume that every amino acid can take 10 different set of bond rotation (a vast understatement perhaps). This means that these 10 conformations can occur independently in EACH of the 100 amino acids in the chain. In other words, there are 10100 DIFFERENT conformations in the chain. This is a whopping BIG number!. How big is it, you ask? This number has been named it's called a Google! What nerds caleld their company after a number?
Lets also assume that 1010 (that is 10,000,000,000 (10 billion)) new conformations can be tested every second. That's pretty fast! and on about the order of molecular vibration time. This means that it would take about 1090 seconds for this small protein to test every possible conformation.
By the latest estimates, the universe as we know it is about 13 billion (1.3x1010) years old. How many seconds is that?
Our small protein can possibly test
4X1017seconds X 1X1010conformations/second =
About 63 orders of magnitude (90-27) too short for a single protein to guarantee folding into the correct shape!. This does not mean 63 universe lifetimes, but rather 1x1036 universe lifetimes!!
Indeed many proteins fold to their final structure within milliseconds to seconds. There must be some kind of organization to the folding process. The first level of organization to protein folding can be identified in dfferent levels of protein structure.
There are four levels of protein structure.
We will look at each of these in turn and then try to understand some generalizations in how protein are organized.
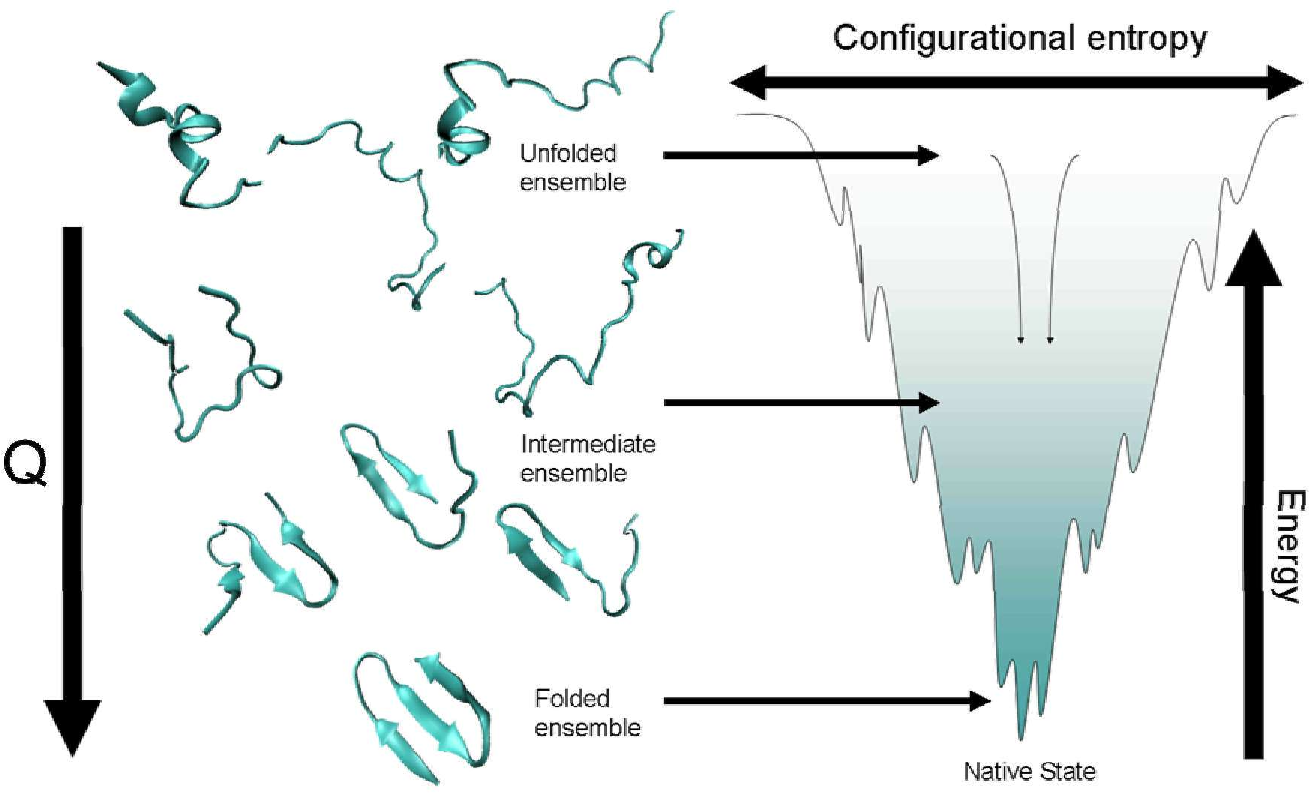
It is all based on energy. Basically nature is lazy. All those interactions we discussed in the previous pages? Each "favorable" interaction between amino acids and water decreases the amount of energy in the protein. Each unfavorable interaction increases the energy of the protein. The end result.. the protein tests a variety of folds until it reaches a conformation that is lowest in energy. This is depicted in the picture to the right. An unfolded protein has a high energy (because of all those hydrophobic amino acid side chains exposed to water etc.) These are at the top of the picture. As the protein begins to test some folds, favorable interactions are made and the energy decreases. This continutes until it reaches a conformation that is lowest in energy (has the most amount of favorable and least amount of unfavorable interactions possible). When the protein gets to this shape, it stays there (kind of) until one puts energy back into it to get it to start unfolding (heat, urea, drastic change in pH, etc.)
It's kind of like going to bed at night. At the start you are at a high energy state (maybe) but then you start to lose energy... you sit, you take off your shoes, put on pajamas, get under the blankets. Each of these favorable interactions lowers your energy state... until you get to the lowest energy possible (while still alive anyway) and fall asleep. Just like that protein you will stay in that lowest energy state (kind of) until one puts energy back into the human (alarm clock, caffeine, breakfast etc.)