
The hydrogen bond (H-bond) is a SPECIAL CASE of polar interaction.
Orientation must be favorable and highly specific. You must be able to draw a "straight line" between the three atoms involved. Note that in the picture below that the covalent the O-H bond is pointing directly toward a lone pair of electrons on the "O" atom of the neighboring molecule. We can draw a straight line through all three of these atoms.
Distance must be short. The distance between the two "O" atoms in the picture below is very short. In fact the distance is actually shorter than that calculated for two "O" atoms and one "H" atom. Since both "O" atoms are somewhat negatively charged, this short distance can occur IF and ONLY IF there is a somewhat positively charged hydrogen atom in the middle.
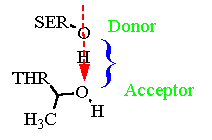 Depicted are the sidechains of a threonine and a serine. Note the orientation of the "O-H" bond of the serine relative to the "O" atom of th threonine.
Depicted are the sidechains of a threonine and a serine. Note the orientation of the "O-H" bond of the serine relative to the "O" atom of th threonine.
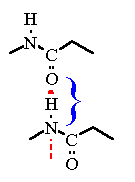 Two Peptide bonds are represted. Note how the "N-H" bond of the "bottom" group is pointing right at the Oxygen atom of another peptide bond.In this picture which is the H-bond donor and which is the H-bond acceptor?
Two Peptide bonds are represted. Note how the "N-H" bond of the "bottom" group is pointing right at the Oxygen atom of another peptide bond.In this picture which is the H-bond donor and which is the H-bond acceptor?
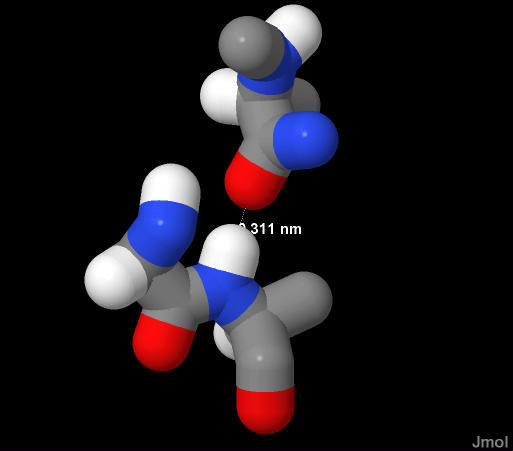 Represetnted here are two peptide bonds. The blue nitrogen of the "lower" amide very close to the red oxygen of the "upper" amide. It is easy to see the N-H bond points directly at the "O" of the neighboring amide.
Represetnted here are two peptide bonds. The blue nitrogen of the "lower" amide very close to the red oxygen of the "upper" amide. It is easy to see the N-H bond points directly at the "O" of the neighboring amide.
Recall that the amide bond (peptide bond) is also polar! H-bonds between peptide bonds are very common (See helices and sheets)