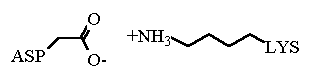 Recalling that opposites attract we see that there is potential for favorable interactions between members of opposite charges. The picture here shows an example of a favorable interaction between the sidechains of an ASP and a LYS.
Recalling that opposites attract we see that there is potential for favorable interactions between members of opposite charges. The picture here shows an example of a favorable interaction between the sidechains of an ASP and a LYS.
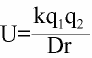 U = the "strength" of the ionic incteraction
U = the "strength" of the ionic incteractionIn the previous module we discussed that several amino acid side chains can carry a formal charge (at least at some pH ranges). Further we found that some amino acids (ASP GLU) can be negative and that others (HIS,ARG,LYS) can be positively charged. Ionic interactions can be quite long range since the magnitude of the interaction depends on the size of the charge (integer values here only for this type), as well as the inverse of the distance between them and some fudge factor that is called the dielectric constant of the solvent.
The dielectric constant is an important property of the solvent since it is a measure of the solvents polarity. The more polar the solvent the stronger the interaction between the solvent molecules and that of the ions. thus polar solvents have the effect of attenuating (shielding) some of the interactions between ions. The dielectric constant for water is 80 while that for a protein is variable but generally considered to be in the range of 2-6.
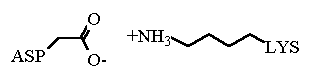 Recalling that opposites attract we see that there is potential for favorable interactions between members of opposite charges. The picture here shows an example of a favorable interaction between the sidechains of an ASP and a LYS.
Recalling that opposites attract we see that there is potential for favorable interactions between members of opposite charges. The picture here shows an example of a favorable interaction between the sidechains of an ASP and a LYS.

At first look this would appear as though ionic interactions would be very strong and important factors in maintaining proteins structure. But in fact these are RARELY seen on the INTERIOR of proteins. They are generally found at the surafce of a protein (at the protein water interface) or between protein subunits. (portions of a protein that are made from different polypeptides yet are required to be in contact to maintain an active unit). At the end of this module I will attempt explain this phenomenon.