White=Hydrogen
There is really nothing to add here except to serve as a reminder. This is perhaps the most basic of the interactions. Recall that every atom takes up space. We can treat the atom (for the purposes that we need at least) as if it is a hard sphere, like a billiard ball. That is that two atoms cannot take up the same space at the same time. Further each type atom has a well characterized diameter. Hydrogen atoms are very small; Carbon, Oxygen and Nitrogen atoms are all about the same intermediate size; while Sulfur and Phosphorus atoms are quite large (relatively speaking of course).
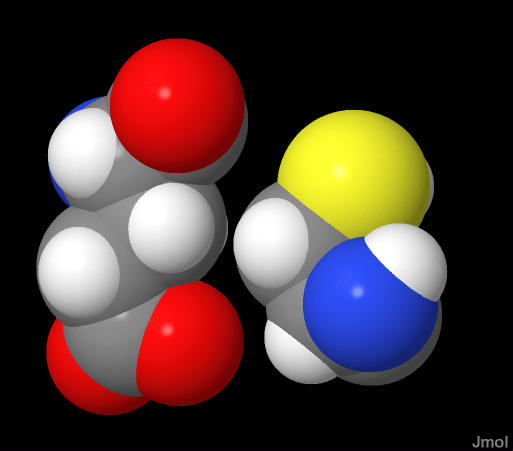
White=Hydrogen
This of course has important implications for protein structure both at the amino acid and the protein. As the atoms of an amino acid are covalently bonded AND take up space, there will be limits on how many shapes and amino acid can have. As the protein folds and amion acids some in contact, there is of course a limit to how "close" they can get. Seen to the left are two amino acids in a folded protein structure shown to demonstrate how close they come to each other.
