
Some proteins are not "water soluble" but rather are embedded in whole or in part in the membrane. This has very significant implications for the structure and the function of the protein
The generalization about the location of nonpolar amino acid sidechains from the previous page now is no longer 100% valid. Because a region of the protein that is in contact with the hydrophobic center of a membrane now has little "incentive" to have them all buried. Indeed, hydrophobic amino acids are observed on the exterior of the protein in these regions.
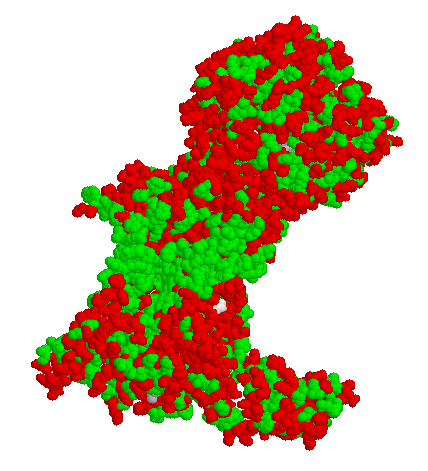 Shown above is protein in the oxidative phosphorylation pathway (more about this in module 7) that is membrane bound. In the initial view, all atoms of the protein are shown in "spacefilling model" and they are colored green for nonpolar amino acids while the polar amino acids are colored red. Notice that in a horizontal band near the center there is a distinct STRIPE of green. This is region of the protein that transects the membrane. The "top and bottom" of the protein are exposed to water.
Shown above is protein in the oxidative phosphorylation pathway (more about this in module 7) that is membrane bound. In the initial view, all atoms of the protein are shown in "spacefilling model" and they are colored green for nonpolar amino acids while the polar amino acids are colored red. Notice that in a horizontal band near the center there is a distinct STRIPE of green. This is region of the protein that transects the membrane. The "top and bottom" of the protein are exposed to water.
The remaining conclusions from the previous page, however, still hold. There are still very few ionic interactions found in the interior of a protein that is membrane bound. The factors that kept them out of the center of a water soluble protein are still in play here. (Ionic groups just don't want to be out of water.) There are sill H-bonds between polar groups (peptide bonds) buried within the protein and the Van Der Waal's forces still apply of course.
|
|