
Elements of Protein Structure
Quaternary Structure
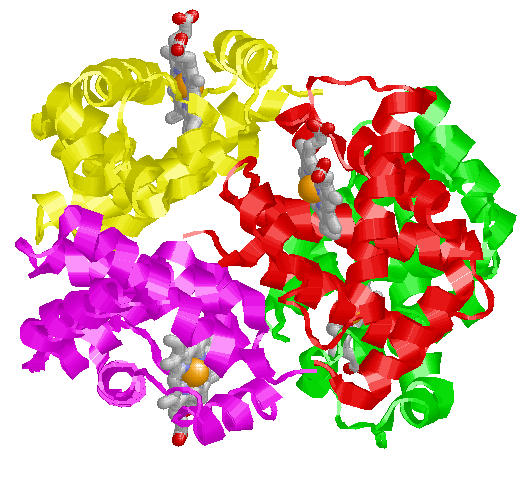 This picture shows a protein that requires 4 subunits to form a functional protein. In this case, the protein is hemoglobin (the oxygen carrying protein in blood). Each subunit is represented in the ribbon models (no amino acid atoms are shown) and colored differently. Subunit A is colored red; B is green; C is magenta; D is yellow. The subunits are transcribed and translated independently. They associate into a permanent complex to form a functional hemoglobin molecule. The atoms that are shown are the heme molecules. The cofactors in these proteins with which molecular oxygen actually associates to be carried from the lungs to the tissues. The red balls inside each heme molecule is the essential Iron atom.
This picture shows a protein that requires 4 subunits to form a functional protein. In this case, the protein is hemoglobin (the oxygen carrying protein in blood). Each subunit is represented in the ribbon models (no amino acid atoms are shown) and colored differently. Subunit A is colored red; B is green; C is magenta; D is yellow. The subunits are transcribed and translated independently. They associate into a permanent complex to form a functional hemoglobin molecule. The atoms that are shown are the heme molecules. The cofactors in these proteins with which molecular oxygen actually associates to be carried from the lungs to the tissues. The red balls inside each heme molecule is the essential Iron atom.
Some proteins require more than a single polypeptide to be a single functional unit. How these multiple polypeptides are arranged describes the quaternary structure. Each of the polypetides has its own independent set of primary, secondary and tertiary structures. Then the polypeptide assemble to generate the functional unit. Since the polypeptides are translated independently, and then fold independently, they do not weave together but rather come together much like a tennis ball and a Velcro racquet.
The use of the Velcro analogy again is on purpose. These polypeptides are "held" together by the same set of interactions that are important in maintaining tertiary structure.
- Steric interactions
- Ionic interactions
- Polar interactions/ H-bonds
- Van Der Waals Forces
- Hydrophobic effects
- Occasionally covalent bonds between sidechains (as with tertiary structure these do not drive the association, but rather stabilize one it has formed).
There will be much more about how the subunits act together for form a functional unit as the course progresses. But for now I will add that they form for different reasons. Sometimes the various subunits within the protein have very different functions - each performing a part of the overall reaction (for instance). In these cases, only the total reaction of all the subunit combined make sense. we will see many examples of this throughout the metabolism portion of the course. In other cases all the subunits have the same function, but they "cooperate" in specific ways to enhance the abilities of the activity. As we will see in module 8, this most frequently occurs when the apparent rate of an enzyme must change to accomodate varying physiological states (regulation).
Interactions Between Subunits
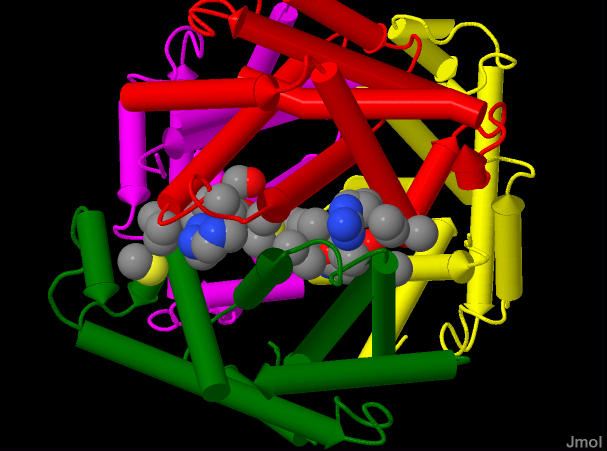 This is an image of hemoglobin showing all four subunits colored as described above, but with a few of the amino acid sidechains shaown as sheres - colored by element. The sidechains shown are those from either the A or B subunit that come in contact with each other. These are responible for how the subunits associate
This is an image of hemoglobin showing all four subunits colored as described above, but with a few of the amino acid sidechains shaown as sheres - colored by element. The sidechains shown are those from either the A or B subunit that come in contact with each other. These are responible for how the subunits associate
| |
| Hemoglobin has four subunits but only two different types. one type is called α and the other β. the common notation is that hemoglobin has an α2β2 configuration. There is a high degree of symmetry to how the protein assembles. Below three different interfaces between subunits: The A-B interface, the B-C interface and the A-C interface are shown. Between some hydrophobic effects are the major type while in others polar interactions dominate. This ismeant to look in different steps click on 1&2 and then 3. uncheck all three then do 4&5 and then 3. There tend to be more cases of ioninc interactions between subunits than can be found in teh interior of a folded polypeptide. There are a couple examples of ionic interactions shown below between the A and C as well as the A and D subunits. |
|
|
|

|
|
Click an atom to diplay it's identity here
|
|
Messages about the currently highlighted features
|
rotate molecule left mouse button, drag
rotate molecule (Z-axis only) Shift + right mouse button ←→
Zoom in/out Shift + left mouse button ↑↓
Move molecule Crtl + right mouse button ↑↓
Java menu right mouse button
|

 This is an image of hemoglobin showing all four subunits colored as described above, but with a few of the amino acid sidechains shaown as sheres - colored by element. The sidechains shown are those from either the
This is an image of hemoglobin showing all four subunits colored as described above, but with a few of the amino acid sidechains shaown as sheres - colored by element. The sidechains shown are those from either the