
In module 1 we discussed that the properties of the amide bond were such that:
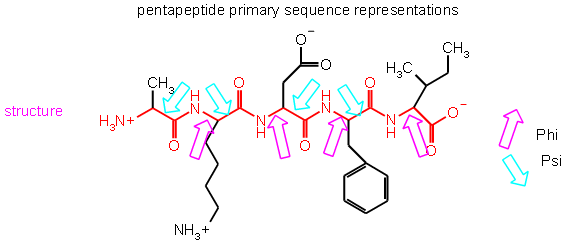
The lack of rotation about the C-N bond is particularly important in discussing the secondary structure of a protein. There are only three different bonds in the backbone and one of them (the amide) does not allow free rotation. The other two bonds: N-Cα and the Cα-C bonds called Phi(φ) and Psi(ψ), respectively are single bonds and do allow "free rotation". In the graphic above, φ is pointed to with the magenta arrows and ψ is pointed to with the cyan arrows. This is where the backbone can bend so that it can form into different structures discussed in the next couple pages.
I put the "free rotation" in quotes because there are some caveats to this. As these bonds are rotated the atoms that they connect are changing relative position. In some of these positions, we find that the the connecting atoms collide (steric interaction). When this occurs we find that some angles of φ and ψ are "not allowed" due to this collision of atoms. In other positions, however, there are no collisions between atoms and these are allowed. On the next page you should be able to rotate φ and ψ (somewhat) independently. As you do this try to see where the steric conflicts arise. Look particularly at the atoms of the sidechains and how these collide with the backbone atoms.
How is an angle of rotation about a bond mesured? This is called a
On the next page, there is a graph of φ vs. ψ and a region shaded purple that are the allowed regions of these angles. Look in particular as to why only the left side of the graph is allowed. What is preventing the angles (shaded pink) on the right from being allowed in most amino acids... except glycine.